37 energy level diagram chemistry
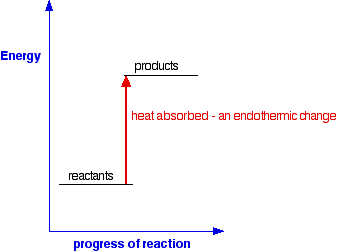
Rates of Reaction. What are Energy Level Diagrams?. The change in energy of a chemical reaction can be plotted against its progress as the reactants turn into products.The energy level diagram for an exothermic reaction is shown below.. Going from the reactants to the top of the curve, we are going up the energy axis of the graph. Energy is being put in to break bonds in the reactants. In this video we take a look at energy level diagrams and how we can interpret them. They allow us to see visually whether a reaction is endothermic or exoth...
This is the first time I reblog a post from a fellow computational chemist and the reason why I do it is because of its beautiful simplicity and usefulness. Given the scope this blog has taken I think this post becomes most appropriate. This post will show you how to create an energy level diagram using nothing but MS Excel.
Energy level diagram chemistry
The electrons surrounding an atom are located in regions around the nucleus called "energy levels". An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be. The first energy level is closest to the nucleus. The second energy level is a little farther away than the first. The energy levels are tracked using an enthalpy diagram. An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to ... A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium
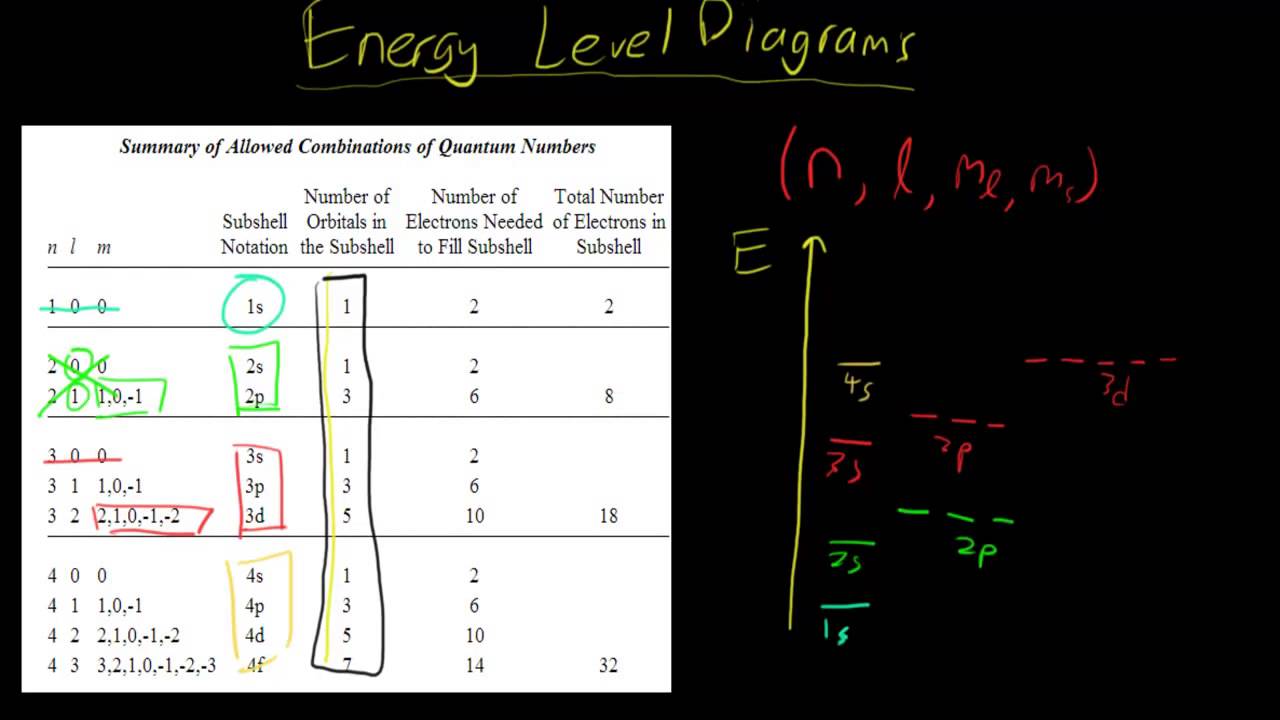
Energy level diagram chemistry. The molecular energy levels are labelled by the molecular term symbols. The specific energies of these components vary with the specific energy state and the substance. Energy level diagrams. There are various types of energy level diagrams for bonds between atoms in a molecule. Examples The outermost principal energy level is of great interest in chemistry because the electrons it holds are the furthest away from the nucleus, and therefore are the ones most loosely held by its attractive force; the larger the distance between two charged objects, the smaller the force they exert on each other. Energy Level Diagrams. Energy level diagrams (sometimes called reaction profiles) are graphical representations of the relative energies of the reactants and products in chemical reactions. The energy of the reactants and products are displayed on the y-axis and the reaction pathway is shown on the x-axis. Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams. Important observations from energy level diagrams of multi electron atoms are: 1)The sub shell of a particular shell do not have equal energies.For Ex: 2s and 2p have different energies.
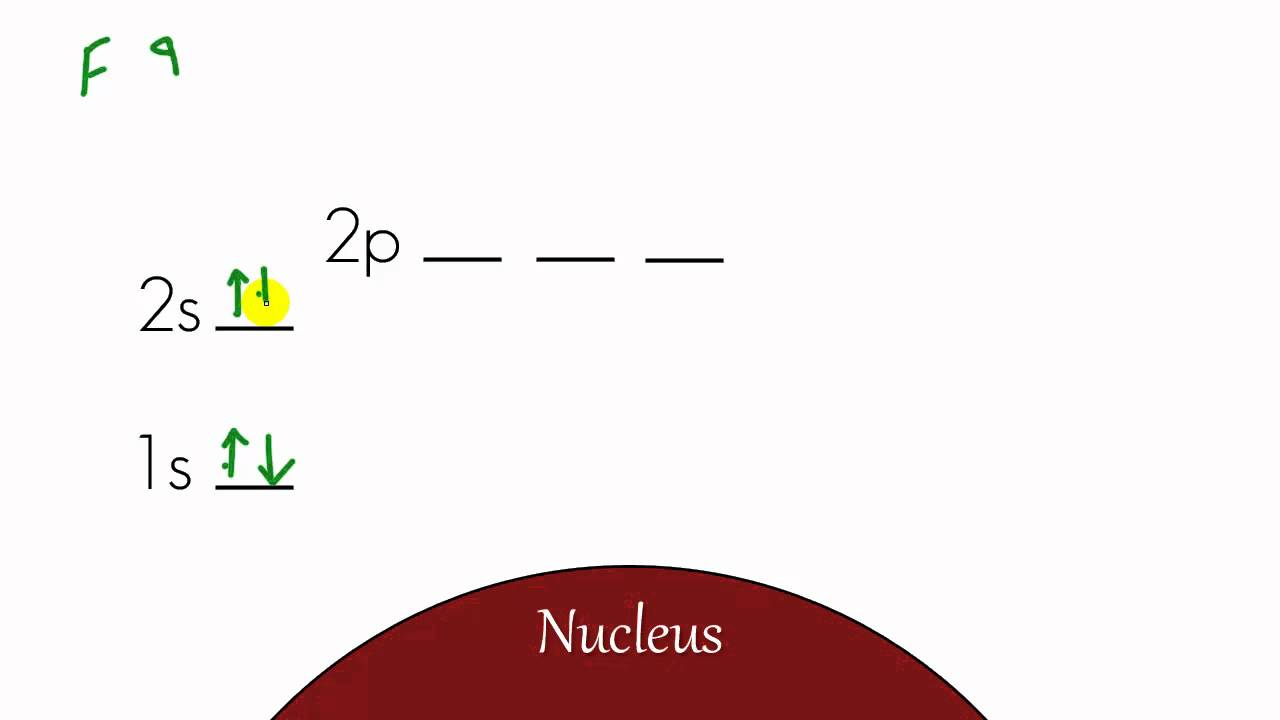
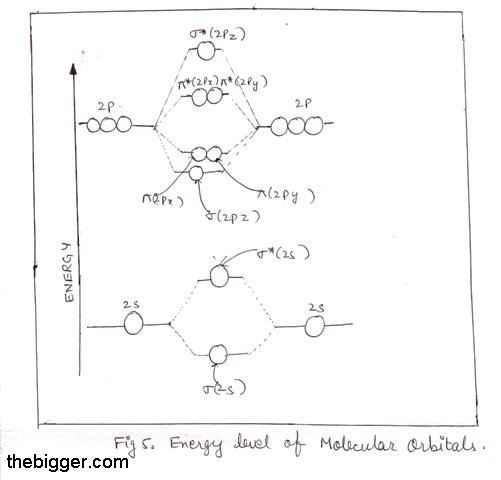
Aufbau diagram for lithium. The electron configuration of lithium, shown on an Aufbau diagram. The following steps detail how to draw an Aufbau diagram: Determine the number of electrons that the atom has. Fill the s orbital in the first energy level (the 1s orbital) with the first two electrons. Filed Under: Chemical Bonding and Molecular Structure, Chemistry, Class 11 Tagged With: bond order, Energy level diagram for Molecular orbitals About Mrs Shilpi Nagpal Author of this website, Mrs Shilpi Nagpal is MSc (Hons, Chemistry) and BSc (Hons, Chemistry) from Delhi University, B.Ed (I. P. University) and has many years of experience in ... photoelectron spectroscopy, the above two energy levels may be written alternatively as 3p 1/2 and 3p 3/2. Specifying the energy levels with Auger notation involves using the letters K, L, M,… for the principal quantum number 1, 2, 3,… and a subscript that depends on the orbital quantum number ( ) and the total angular momentum quantum ... Re: H-Atom Energy Level Diagram. Hi! The energy of electrons can only have certain values. This corresponds to the energy levels n=1, n=2, n=3, etc. When an electron absorbs a specific wavelength of light that matches the energy needed to move, it can jump levels. An electron at a higher level with high energy will eventually drop back to a ...
An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction with time; The transition state is a stage during the reaction at which chemical bonds are partially broken and formed What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus. The shells can be denoted by alphabets (K, L ... The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. Note that moving left or right on an energy level diagram doesn't actually represent anything ... Energy Profiles (Energy Diagrams) Chemistry Tutorial Key Concepts. An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction.
Energy level diagrams. are used to model energy. changes during reactions. They show the relative energy levels of the products and reactants . Exothermic reaction
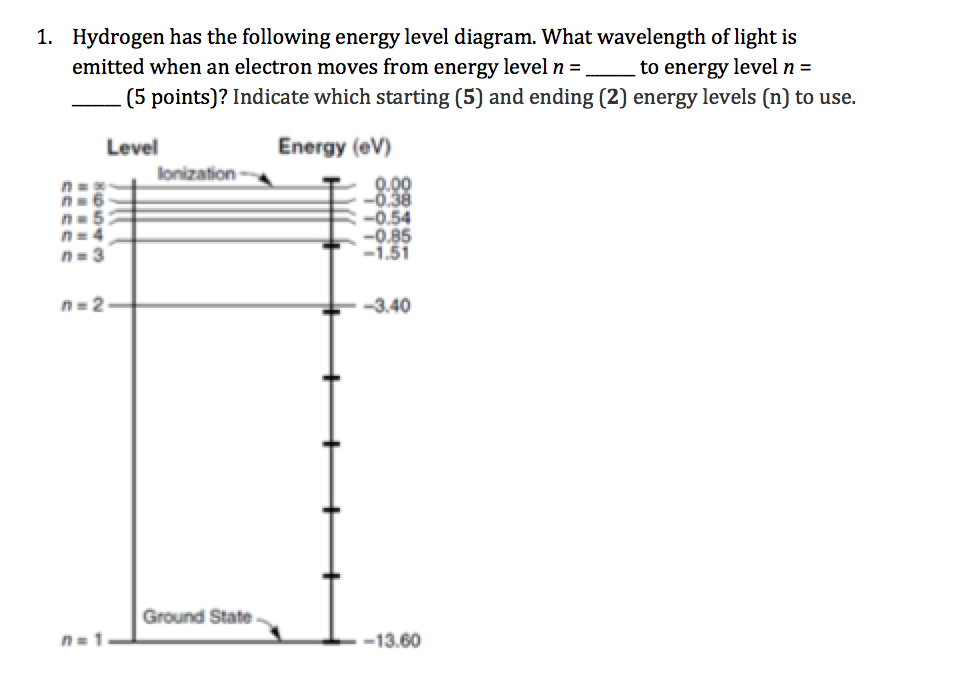
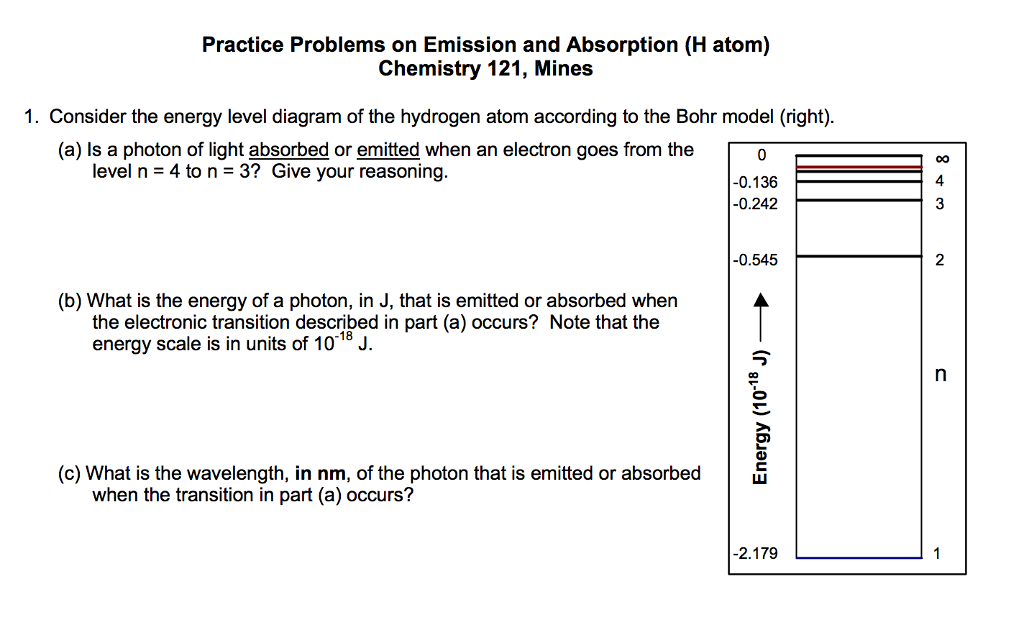
Total energy of an electron in Bohr’s nth stationary orbit is: E n = - \[\frac{2πmK^{2}Z^{2}e^{4}}{n^{2}h^{2}}\] ….(5) Or, E n = - \[\frac{13.6}{n^{2}}\] …(6) Here, TE of an e-in a stationary orbit is negative, which means the electron is tightly bound to the nucleus. Energy Level Diagram [Image will be Uploaded Soon]
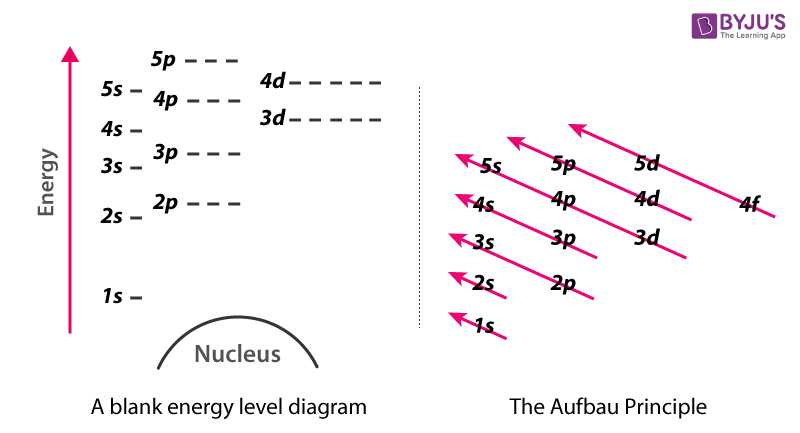
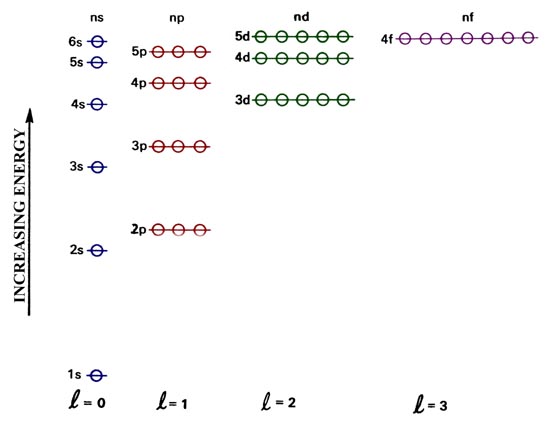
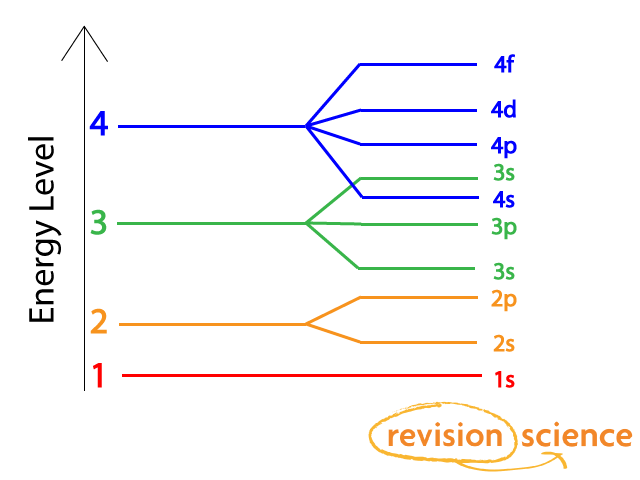
The Aufbau principle tells you that the lowest-energy orbitals fill first, but the specific order isn't sequential in a way that's easy to memorize. See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n = 3 level only has s, p and d orbitals.
Chemissian is an analyzing tool of the molecule electronic structure and spectra. It allows one to build and analyze molecular orbital energy-level diagrams (Hartree-Fock and Kohn-Sham); analyze calculated experimental UV-VIS electronic spectrum and compare it with experimental one on the same plot; calculate and visualize natural transition orbitals, electronic and spin densities and prepare ...
Chemistry Concepts: Energy Levels and Orbitals. A lot of chemistry is explained by the sharing and trading of electrons between atoms. Understanding how electrons are arranged in an atom is a building block of Chem I. Electrons in an atom are contained in specific energy levels (1, 2, 3, and so on) that are different distances from the nucleus.

Figure 7 J Aggregates Of Amphiphilic Cyanine Dyes For Dye Sensitized Solar Cells A Combination Between Computational Chemistry And Experimental Device Physics
Chemists sometimes use an energy level diagram to represent electrons when they're looking at chemical reactions and bonding. An energy level diagram is more useful and easier to work with than quantum numbers in the quantum mechanical model. Chemists use the energy level diagram as well as electron configuration notation to represent which energy level, […]

Jasper Green On Twitter Go Deep Or Go Home Activation Energy And Energy Level Diagrams Https T Co I3jhmp7rty Chemistry Rsc Eic
Students will use data from the lab to model and explain the phenomena using energy level diagrams. For the task that follows, students will be given temperature data from a chemical reaction and, based on these data, students relate the change in temperature to the energy associated with chemical reactions in order to determine whether a reaction is exothermic or endothermic.
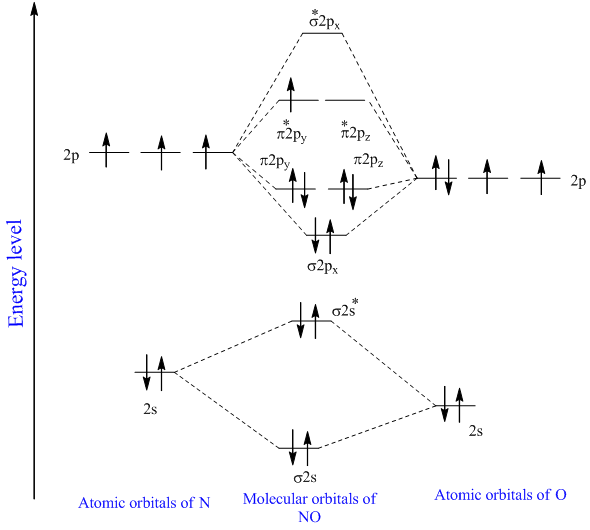
As can be seen from the energy diagram - four of the molecular orbitals. Generate the Molecular Orbitals for CH4 (Td), CH4 (D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us. Here is an energy level diagram showing ...
Photosynthesis, the process by which green plants and certain other organisms transform light energy into chemical energy. During photosynthesis in green plants, light energy is captured and used to convert water, carbon dioxide, and minerals into oxygen and energy-rich organic compounds.

The Energy Level Diagram Shown Here Can Be Continued To Higher Energies The Next Few Orbitals In Homeworklib
Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. It only takes a minute to sign up. Sign up to join this community. ... Nice Energy Level Diagrams with rxnlvl - wp.me/ptBY1-2W $\endgroup$
Energy level diagram is a part of CBSE Class 11 chemistry first term syllabus. It carries a total of 12 periods and 5 to 6 marks. Atomic radius value: r(n) = n 2 x r(1)
Enthalpy level diagrams. When a Hess' law energy cycle is presented graphically showing the relative chemical energies of the reactants and products, this is called an energy, or enthalpy level diagram. Personally, I find these more logical than Hess' energy cycles, however it's a matter of individual preference.
Chemistry 2017. DAY ONE: October 3 ... •In each energy level, electrons fill sublevels in a certain order Level 1: a) only has one s sublevel (a spherical shape) ... diagram. Order of Orbitals—Periodic Table For the d block n-1 For the f block n-2. Hotel analogy video s s s s p d d p p f These are 4 levels each
Nonradiative transitions arise through several different mechanisms, all differently labeled in the diagram. Relaxation of the excited state to its lowest vibrational level is called vibrational relaxation. This process involves the dissipation of energy from the molecule to its surroundings, and thus it cannot occur for isolated molecules.
D3.3 Orbital Energy Level Diagrams An orbital energy level diagram (or just orbital diagram) shows the relative energies of orbitals and how electrons are distributed among orbitals within a subshell.In an orbital energy level diagram, individual orbitals are usually represented by horizontal lines whose vertical position conveys the qualitative relative energies of the orbitals.
Nov 21, 2010 · The ionization energy of an atom is the energy required to remove the electron completely from the atom.(transition from ground state n = 0 to infinity n = ∞). For hydrogen, the ionization energy = 13.6eV; When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. The Lyman(ultraviolet) series ...
Chemistry Lesson 3.2Energy Level DiagramsPauli Exclusion PrincipleHund's RuleAufbau PrincipleDiagonal RulePractice problems#energyLevelDiagram #quantum #ketz...

Answer The Following Question Draw A Qualitatively Energy Level Diagram Showing D Orbital Splitting In The Octahedral Environment Predict The Number Of Unpaired Electrons In The Complex Chemistry Shaalaa Com
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium
The energy levels are tracked using an enthalpy diagram. An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to ...
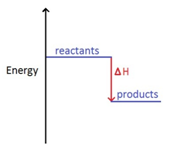
3 05 Triple Only Draw And Explain Energy Level Diagrams To Represent Exothermic And Endothermic Reactions Tutormyself Chemistry
The electrons surrounding an atom are located in regions around the nucleus called "energy levels". An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be. The first energy level is closest to the nucleus. The second energy level is a little farther away than the first.















0 Response to "37 energy level diagram chemistry"
Post a Comment