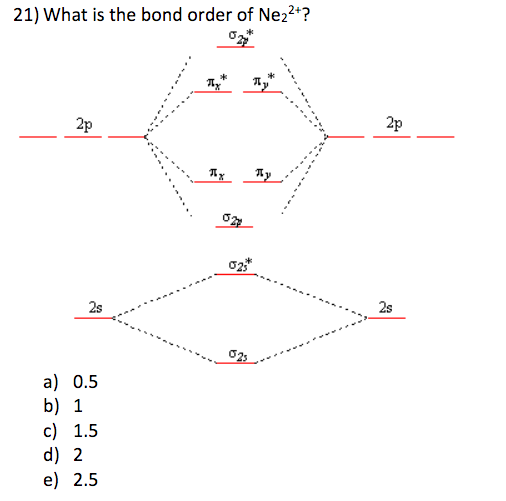
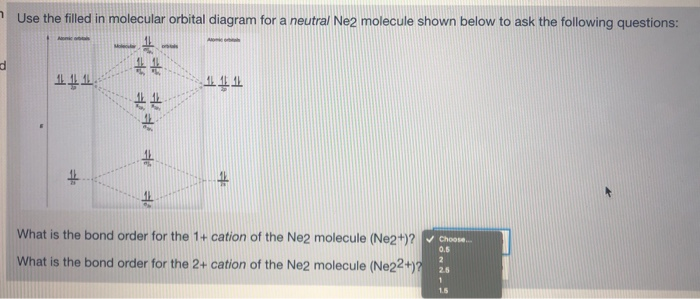
37 molecular orbital diagram for ne2 2+
This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Complete Solutions Manual General Chemistry Ninth Edition ... - ID:5dcdb97adce08. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION Ebbing/Gammon. Uploaded by. Sofia Uribe Sanchez. connect to do...
01.05.2021 · Molecular Orbital Theory. Kossel-Lewis Approach to Chemical Bonding: All noble gases [except He] have 8 electrons in their valence shells. They are chemically inactive. In the case of all other elements, there are less than 3 electrons in the valence shells of their atoms, and hence they are chemically reactive. “The atoms of different elements combine with each other in order to complete ...

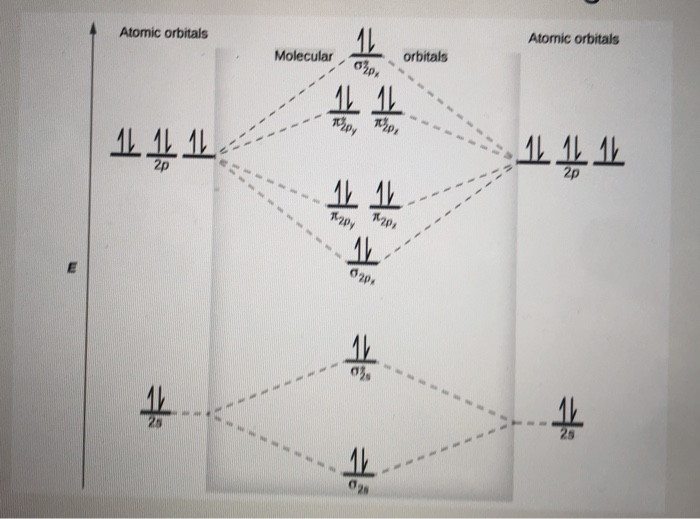
Molecular orbital diagram for ne2 2+
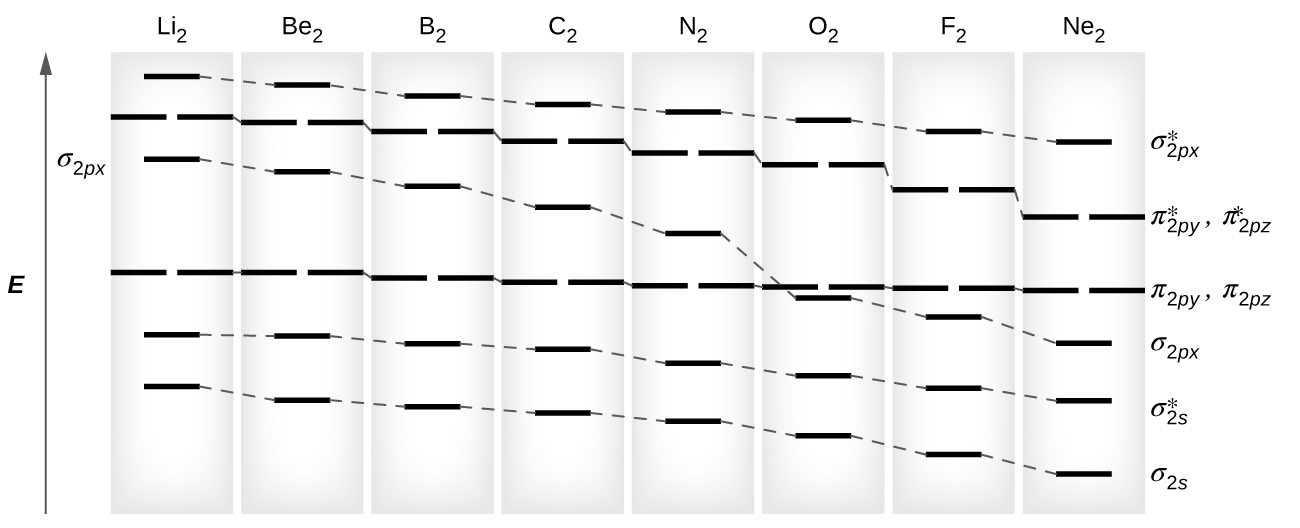
Molecular orbital diagram for ne2 2 To form the 2+ ion, the uppermost electrons in the sigma* 2p orbital are removed, making it isoelectronic with F2, so it has a bond order of 1 and should be ...1 answer · 0 votes: Question: Is Ne2 2+ paramagnetic or diamagnetic? To understand this answer you have to know ... Molecular Orbital Diagram of N 2 Chapter 9 Section 6 Ne2 molecular orbital diagram. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular.
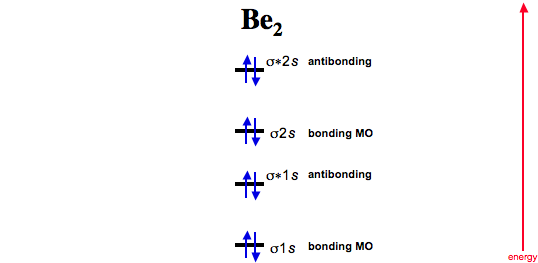
Molecular orbital diagram for ne2 2+. 5:20There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up ...27 Feb 2013 · Uploaded by chemistNATE Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable. With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). ... 24.1k views. asked Jan 26, 2020 in Chemistry by SurajKumar (66.2k points) With the help of molecular orbital diagram show that Ne 2 cannot exist as stable molecule. (Atomic number of Ne = 10). chemical bonding; molecular ... Molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you get sigma2s2sigma2s2pi2p4sigma2p2. A partial molecular orbital energy level diagram for the hf molecule. Which one do i use. I can draw be2 but not this. The bond order is 25.
4 Mar 2018 — Explanation: Neon atom has 10 electrons and its electronic configuration is . When molecule is considered, it has two neon atoms and thus is ...2 answers · 18 votes: Bond order of Ne2 = (10-10) =0 So Ne2 is unstable ;Ne2 cannot exist Answer (1 of 4): Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π*2Py²σ*2Pz² Hence the bond order of Ne2 according to M.O.T is =(Nb-Na)/2 =(10-10)/2 (since,both bonding orbitals and non-bonding orbital contains 10 electrons) =0 Hence, no bond is possible b... A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set. B) A bond order of 0 represents a stable chemical bond. C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate ... Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
2:45Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get ...9 Jun 2017 · Uploaded by chemistNATE Academia.edu is a platform for academics to share research papers. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Now carbon monoxide’s MO diagram is: Hope it helps :) 57.9K views · View upvotes · Answer requested by . Arun M V. 99 29. Siddhesh ... 2:36Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)). ... and it is Paramagnetic. sigma2s(2),sigma2s*(2 ...9 Jun 2017 · Uploaded by chemistNATE
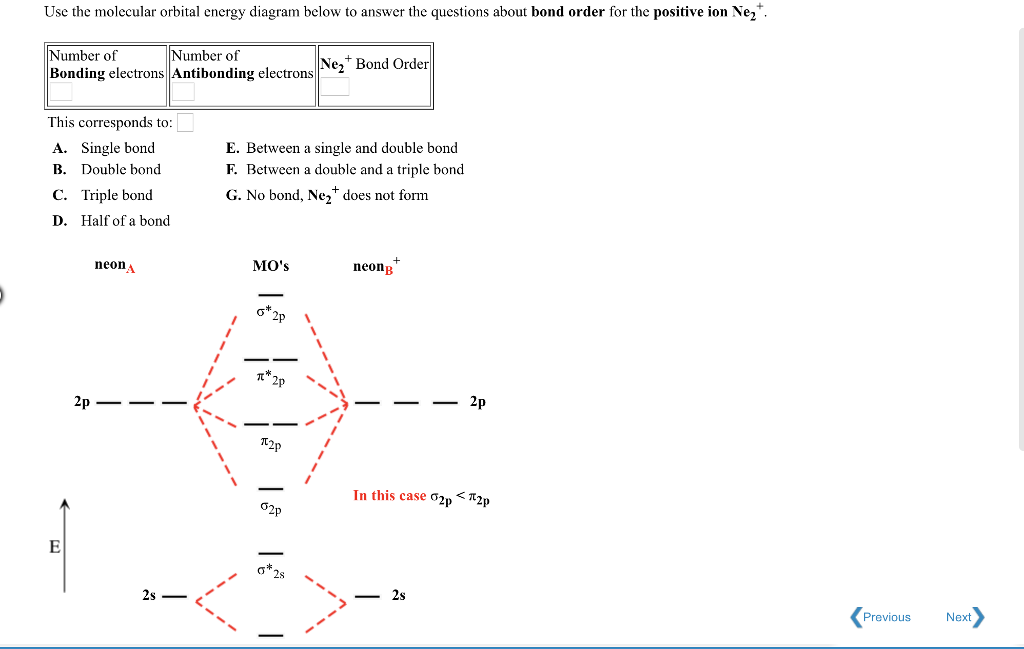
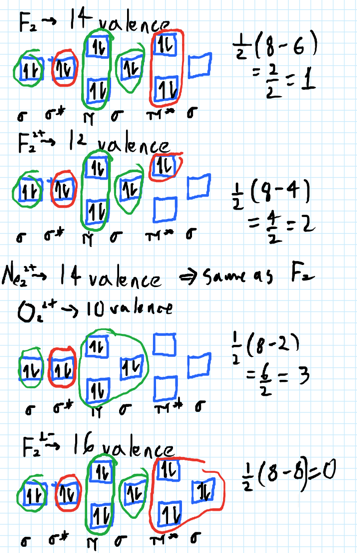
what is the bond order of Ne2 2+. i know its 1 but I do not know how to use this diagram. can someone explain this? Show transcribed image text. Expert Answer.
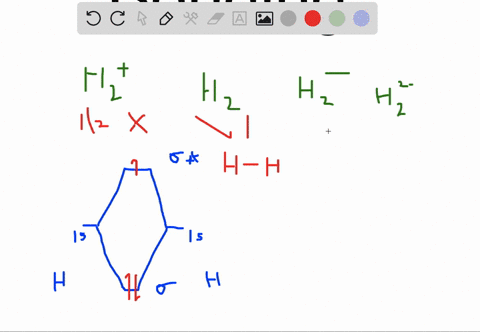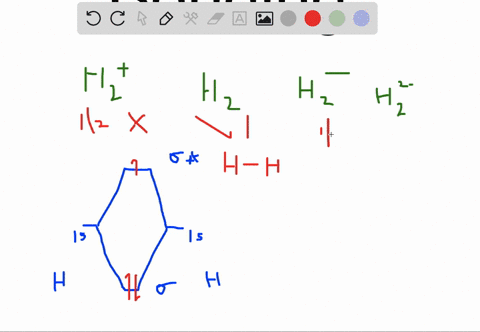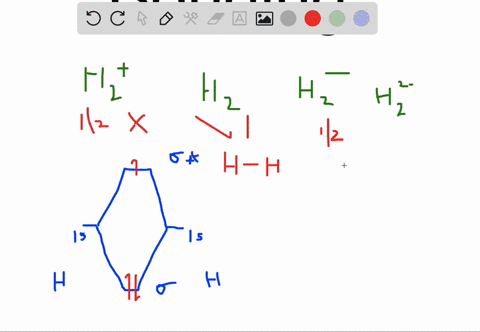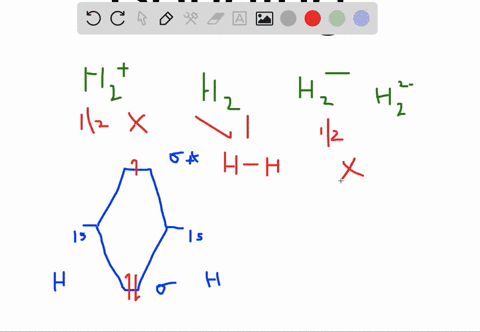
Solved Apply Molecular Orbital Theory To Predict If Each Molecule Or Ion Exists In A Relatively Stable Form A H2 2 B Ne2 C He2 2 D F2 2
Choose the orbital diagram that represents the ground state of N. (a) 1s (2) 2s (2) 2p (6) ... H2^2-Ne2 He2^2+ F2^2-H2^2- : will not exist Ne2 : will not exist He2^2+ : will exist F2^2- : will not exist. Apply molecular orbital theory to predict which species has the strongest bond. (a) O2+ (b) O2-(c) O2 (d) All bonds are equivalent according to molecular orbital theory. O2+ Recommended ...
7:11For formation of oxygen molecule, two oxygen atoms come close and their atomic orbitals overlap to make ...15 Jun 2020 · Uploaded by Edmerls
The Energy Of S2pz Molecular Orbital Is Greater Than P2px And P2py Molecular Orbitals In Nitrogen Molecule Sarthaks Econnect Largest Online Education Community
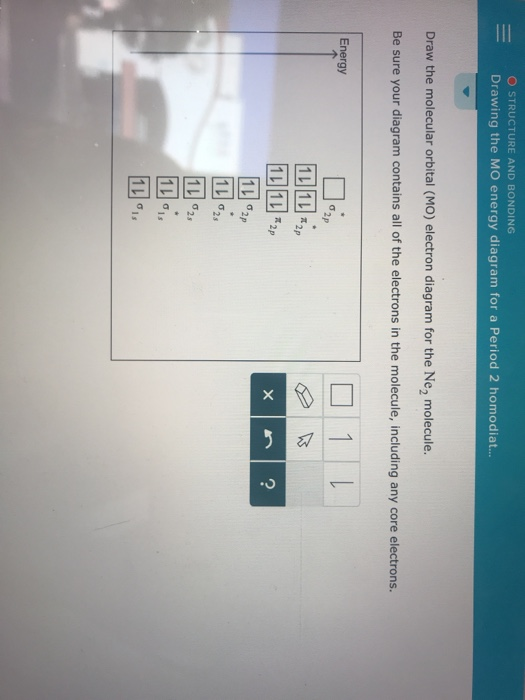
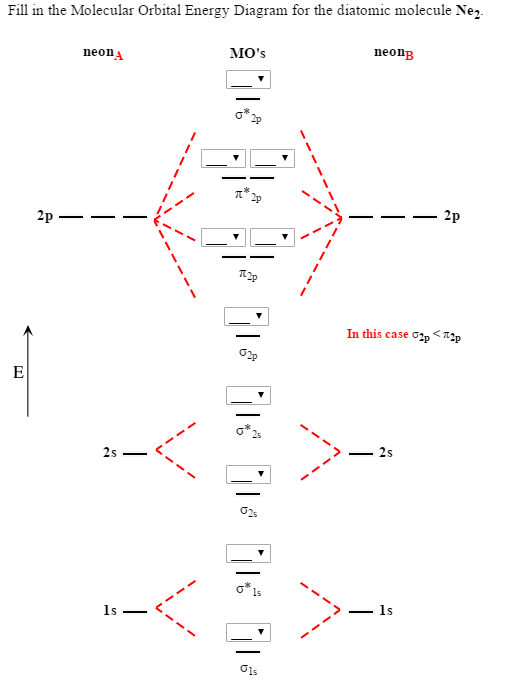
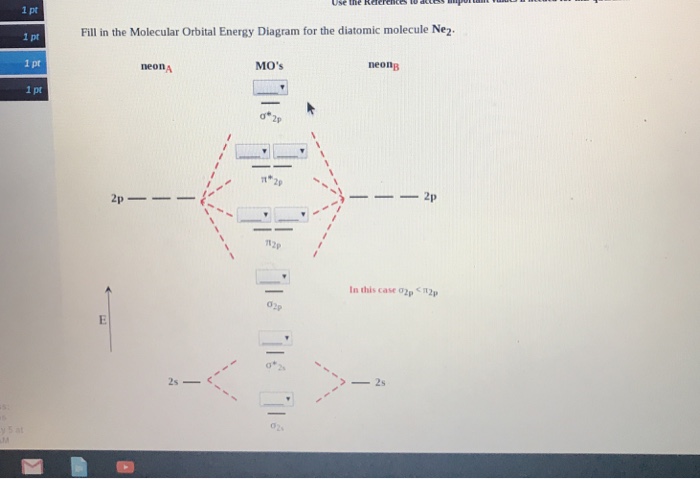
We review their content and use your feedback to keep the quality high. 86% (7 ratings) Transcribed image text: Draw the molecular orbital (MO) electron diagram for the Ne2 molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Energy 1 х 5 ?.
the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
2 3kB T u = m 2 where is the lattice vibrations frequency (1013 1014 s1 ). Thus, kB T G2 Isc = I0 exp . (1.30) m 2 According to quantum mechanics, even at zero temperature there are zero-point vibrations with3. 2 3~. u = 2m In this case ~G2 Isc = I0R exp (1.31) 2m where I0R is the intensity for a rigid
For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. Give each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order. Rationalize the trend in bond order in terms of bond strength.
Molecular Orbital Theory: The molecular orbital theory is based on the idea that when two atomic orbitals of similar energy and symmetry overlap, they combine to form a molecular orbital.
9 Molecular Geometry and Bonding Theories 330 9.1 MOLECULAR SHAPES 332 9.2 THE VSEPR MODEL 334 Effect of Nonbonding Electrons and Multiple Bonds on Bond Angles 338 Molecules with Expanded Valence Shells 339 Shapes of Larger Molecules 342. 9.3 MOLECULAR SHAPE AND MOLECULAR POLARITY 343. 9.4 COVALENT BONDING AND ORBITAL OVERLAP 345
Molecular Orbital Diagram of N 2 Chapter 9 Section 6 Ne2 molecular orbital diagram. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular.
To form the 2+ ion, the uppermost electrons in the sigma* 2p orbital are removed, making it isoelectronic with F2, so it has a bond order of 1 and should be ...1 answer · 0 votes: Question: Is Ne2 2+ paramagnetic or diamagnetic? To understand this answer you have to know ...
Molecular orbital diagram for ne2 2

Draw The Energy Level Diagram To Show That N2 Has Triple Bond O2 Has Double Bond F2 Has Single Bond Brainly In

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable A Homeworklib

Draw And Explain The Molecular Orbital Diagram Of Ne2 On The Basis Of Molecular Orbital Diagram Brainly In

Draw The Molecular Orbital Diagram For Ne2 And Determine If The Bond Between The Two Atoms Homeworklib










0 Response to "37 molecular orbital diagram for ne2 2+"
Post a Comment