39 bf3 molecular orbital diagram
CQ molecular orbitals, identify (i) the atomic orbitals (s or p) used to construct the MO, (ii) the type of MO (a or IT), and (iii) whether the MO is bonding or antibonding. [Sections 9.7 and 9.8] EXERCISES Molecular Shapes; the VSEPR Model 9.11 (a) An AB2 molecule is described as linear, and the CQ A—B bond length is specified. molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ...
In bf3 three sp2 hybrid orbitals of boron form sigma bonds with three fluorine atoms. In the excited state one electron from the 2s orbital jumps to the 2p orbital and then the three 2p orbitals and one 2s orbital mix among themselves giving rise to four sp2 hybridized orbitals one of which is unused in bf3.
Bf3 molecular orbital diagram
by DC Ghosh · 2004 · Cited by 27 — Abstract : The formation of the F3B–NH3 supermolecule by chemical interaction of its fragment parts, BF3 and NH3, and the dynamics of ... 02:00 Reducible representation for sigma group orbitals07:12 Reduction of reducible representation20:08 Effect of each symmetry operation on representa... Orbital Diagram For Fluorine Molecular Orbital Diagram For Bf3 Chemistry Stack Exchange. Orbital Diagram For Fluorine Electron Configuration For Phosphorus P. Orbital Diagram For Fluorine Periodic Table Year 10 Unique Orbital Diagram For As Best Fluorine.
Bf3 molecular orbital diagram. Click the Symmetry Operations above to view them in 3D. BF 3 belongs to the D 3h point group and contains;. one C 3 rotation axis. Three C 2 axes perpendicular to the C 3 Axis. 3σ v planes of symmetry,One σ h plane. An S 3 Axis (not illustrated). Pointgroup Flow Chart . D nd | D nh | D n Pointgroups BF3 Molecular Geometry. The molecular geometry of the BF3 is called trigonal planar. The trigonal planar is a central atom that has three bonded atoms around and has no lone pairs of electrons. That is why the bond and lone pairs' total number becomes three, and the bond form angles of 120°. Molecular orbital diagram for BF3. Ask Question Asked 4 years, 4 months ago. Active 1 year, 2 months ago. Viewed 22k times 15 4 $\begingroup$ I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an ... Complete the molecular orbital (MO) diagram of BF3. Assume a ground state electron configuration. Question: Complete the molecular orbital (MO) diagram of BF3. Assume a ground state electron configuration.
Hybridization of BF3 (BoronTrifluoride) Before we get into the hybridization of BF 3 lets us quickly go through and know few details about Boron trifluoride. This chemical compound is an inorganic compound which is colourless but toxic in nature when it is in the gaseous stage. It produces when reacted with moist air. OF– has 14 valence electrons, four in the π2p* orbitals (see the diagram in the ... 5.20 SO3 has molecular orbitals similar to those of BF3 (Section 5.4.6).29 pages Molecular Geometry of BF3 Each molecule has its shape that can be represented after finding out their electrons hybridized in various types of s-orbital and p-orbital like sp2, sp3, sp. As you found the molecule BF3 is sp2 hybridized (in the presence of 3 orbitals) with 1 boron atom and 3 atoms of fluorine. Assume that fluorine 2s orbitals are not involved in the bonding and only consider the 2p orbitals. z. 3 ! The 2p orbital on each fluorine is perpendicular to ...8 pages
Molecular Orbital Diagram. Molecular Orbital Theory, which is used to sketch the MO diagram of any given molecule, is a complex yet important concept of chemical bonding. In quantum mechanics, MO theory deals with spatial and energetic properties of electrons and talks about the LCAO (Linear Combination of Atomic Orbitals) to form MO( Molecular ... Molecular orbital diagram for BF3. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2p (along the bond axis) for F has an irreducible representation of. hybridization and molecular orbital mo theory molecular shapes based on valence electrons lewis dot structures and electron repulsions •molecular orbital theory mo - a molecule is formed by the overlap of atomic orbitals to form molecular orbitals electrons are then distributed into mos a molecule is a collection of nuclei with the orbitals ... Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1. Symmetry adapted linear combinations (SALC) of bonding group atomic orbitals For molecules where the central atom is bonded to more than one other B group such as the
What is the molecular shape of BF3 quizlet? The BF3 molecule must be trigonal planar. Is BF3 a shape? In BF3, the central atom boron contains three valence electrons and each electron is shared by fluorine atoms (as shown in the below figure) which results in three sigma bonds and no lone pairs.
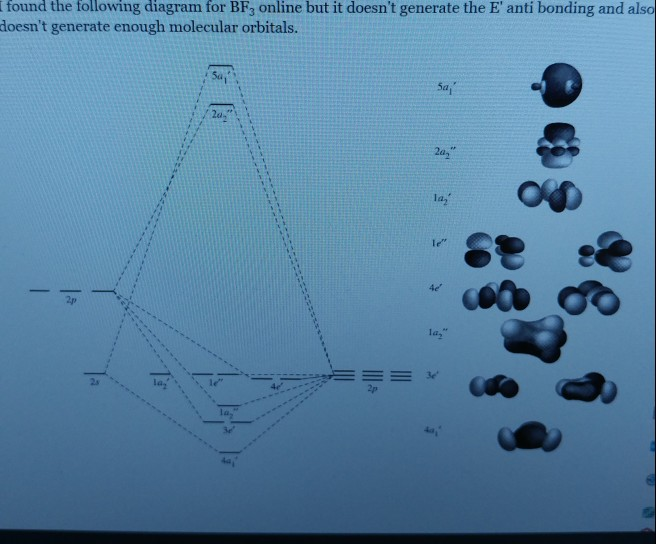
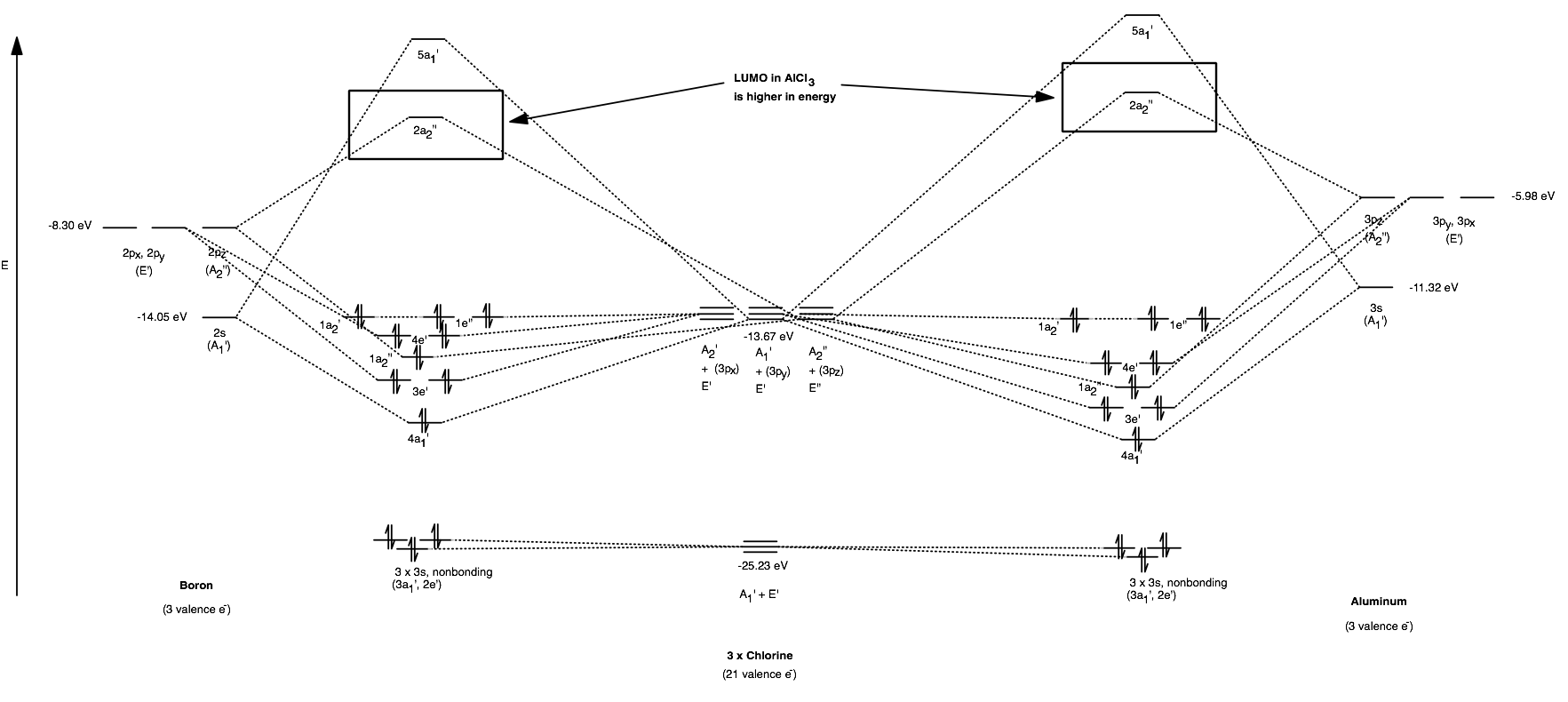
Molecular Orbitals for BF3. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital circle in the energy level correlation diagram shown The energy level diagram may be displayed with or without the group theory symbols and character table: the models accessed by clicking are the same.

Advanced Theories Of Chemical Bonding Theories Of Occupy The Fnof Orbital In H 2 Mo Diagram For H 2 Mar Euro Orbitals Lower Energy Than Fnof Orbital See Mo Diagram B Pdf Document
This photo about: Bf3 Molecular orbital Diagram, entitled as Molecular Orbital Diagram N2 Bf3 Molecular Orbital Diagram - also describes Molecular Orbital Diagram N2 and labeled as: 3 molecular ponents of atp,3 postulates of kinetic molecular theory of matter,3 sulfolene molecular weight,3d molecular model set,worksheet 3 molecular net ionic equations, with resolution 1822px x 2577px
Draw And Explain The M O Diagram Of Boron Molecule Sarthaks Econnect Largest Online Education Community
3.Molecular orbital theory Chapter 5 Bonding in polyatomic molecules Directionalities of atomic orbitals are not compatible with the H-O-H bond angle. 2 Orbital hybridization - sp Hybrid orbitals - generated by mixing the characters of atomic orbitals ( ) 2 1 ...
I'm trying to build a molecular orbital diagram for BF3 and I'm running into problems with irreducible representations on the F side. 2s for B has an ...1 answer · Top answer: I think you are most of the way to the answer, but I will start the process from scratch for the sake of a full explanation for future readers. First, ...
Boron trifluoride is a colorless gas with a pungent odor. It is toxic by inhalation. It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material.Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing.
Transcribed image text: (10 points) Complete the molecular orbital diagram for the tr trifluoride (BF3). Assume each fluorine contributes a single electron from an unhybridized 2p atomic orbital to form a sigma bond with boron via 2p (F) to sp2 (B) overlap.
BF3 is SP2 hybridization. For this molecule, It is SP2 because one π (pi) bond is required for the double bond between the Boron and only three σ bonds are formed per Boron atom. The atomic S - orbitals and P - orbitals in Boron outer shell mix to form three equivalent SP2 hybrid orbitals.
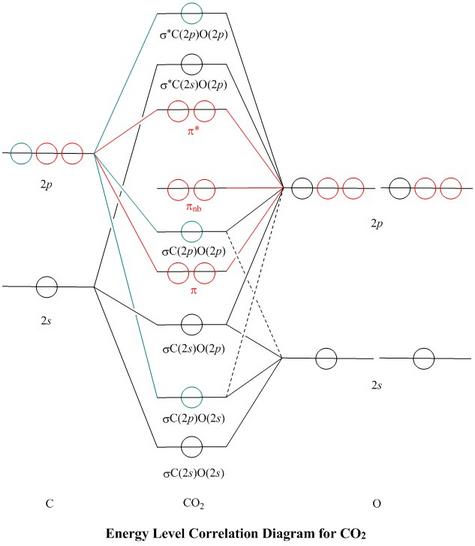
BeF2 uses s and p orbitals on all three atoms, and is isoelectronic with CO2. The energy level. Be able to construct molecular orbital diagrams for homonuclear diatomic, heteronuclear diatomic, homonuclear triatomic, and heteronuclear triatomic molecules. Determine whether the following molecular orbitals are bonding or antibonding.
Molecular Orbital Diagram This is a molecular orbital energy level diagram for the p orbitals. Note that the σ bonding orbital is lowest in energy due to the greater overlap end-onend. σu πg 2p πu σg 2p 29. Molecular Orbital Diagram The alternate notation is provided on the right side of the energy level diagram.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemistry: The Central Science, Chapter 9, Section 5Chapter 9 - Molecular Geometry
Molecular Orbitals for Larger Molecules 1. Determine point group of molecule (if linear, use D2h and C2v instead of D∞h or C∞v) 2. Assign x, y, z coordinates (z axis is principal axis; if non-linear, y axes of outer atoms point to central atom)3. Find the characters of the reducible representationfor the combination of
BF3 is an sp2 hybridization. It is sp2 for this molecule because one π (pi) bond is needed for the double bond between the Boron, and just three σ bonds are produced per Boron atom. The atomic S and P - orbitals in Boron outer shell mix to form three equivalent hybrid orbitals of sp2. The BF3 molecule shape is represented as the BF3 ...
The complete molecular orbital scheme of BF3 is developed here.
Orbital Diagram For Fluorine Molecular Orbital Diagram For Bf3 Chemistry Stack Exchange. Orbital Diagram For Fluorine Electron Configuration For Phosphorus P. Orbital Diagram For Fluorine Periodic Table Year 10 Unique Orbital Diagram For As Best Fluorine.
02:00 Reducible representation for sigma group orbitals07:12 Reduction of reducible representation20:08 Effect of each symmetry operation on representa...
by DC Ghosh · 2004 · Cited by 27 — Abstract : The formation of the F3B–NH3 supermolecule by chemical interaction of its fragment parts, BF3 and NH3, and the dynamics of ...
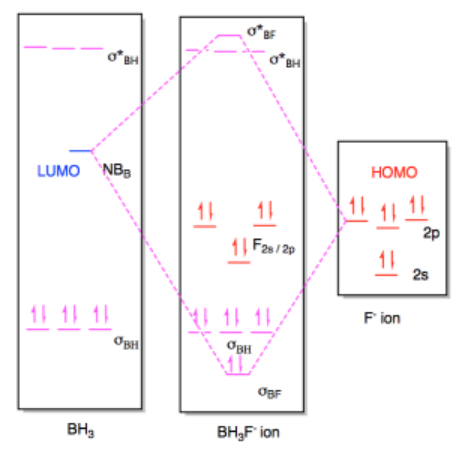
Solved A Calculate And Sketch The Molecular Orbitals Of Bf3 And Nh3 Forming The Bf3 Nh3 Adduct B Examine The Bonding And Anti Bonding Orbitals Involved In The B N Bond In The Bf3 Nh3 Adduct Is

For Ch4 Why Do 2s Orbital And Three 2p Orbitals In The Valence Shell Of Carbon Combine To Form Four Sp3 Hybrid Orbitals Quora

Which Of The Following Is A False Statement About Bf3 A Bf3 Has Trigonal Planar Molecular Geometry B Bf3 Has Trigonal Pyramidal Electronic Geometry C All Three Bond Angles In Bf3 Are



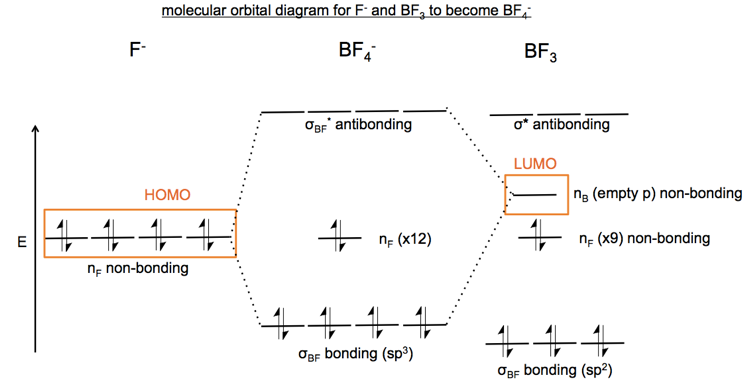


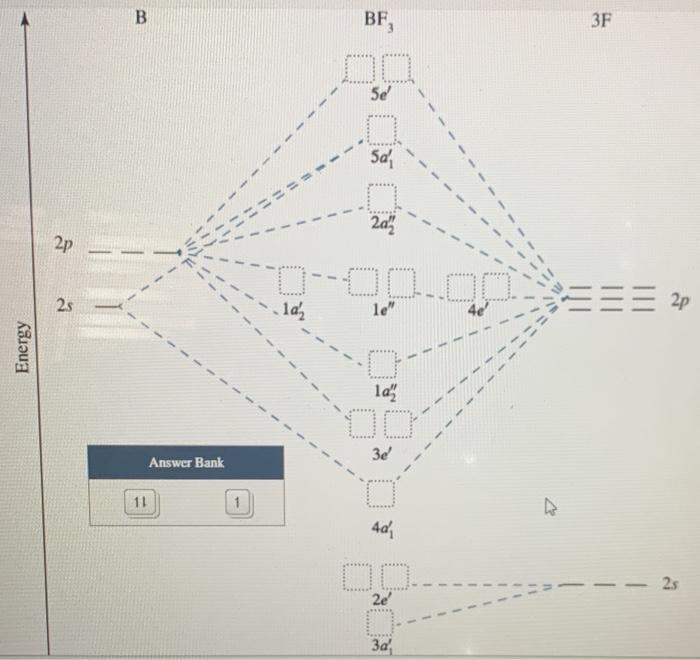




0 Response to "39 bf3 molecular orbital diagram"
Post a Comment