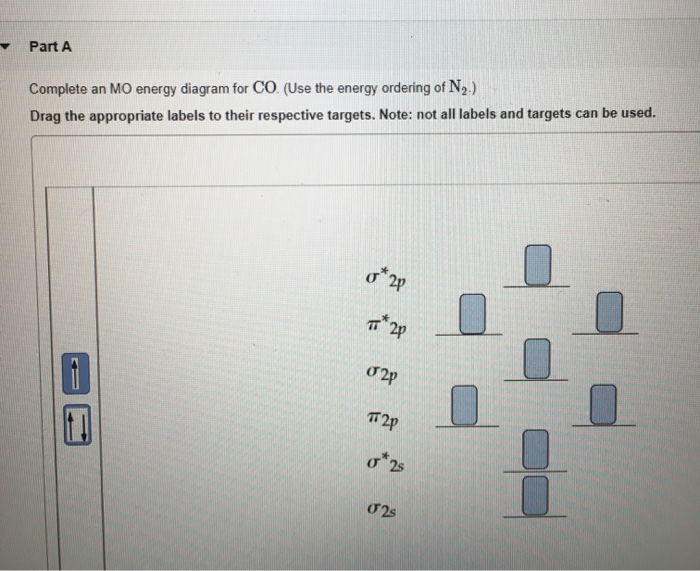
40 complete the mo energy diagram for the n2+ ion.
As said before, all are stable. In addition, all have one or more unpaired electrons. The first of these, incidentally, takes about as much energy to form as a Xe + ion. This is what initially led to the discovery of xenon compounds. Problem 7.93: Use the MO diagram in figure 7.18a to describe the bonding in N 2 +, N 2, and N 2-. Which of the ... (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
Answer (1 of 5): N2+ has less bond energy. This is because, according to molecular orbital theory, it has fewer electrons in bonding orbitals. The diagram above is the molecular orbital diagram for N2. The highest electron on the diagram is the highest energy electron, and thus the electron that...
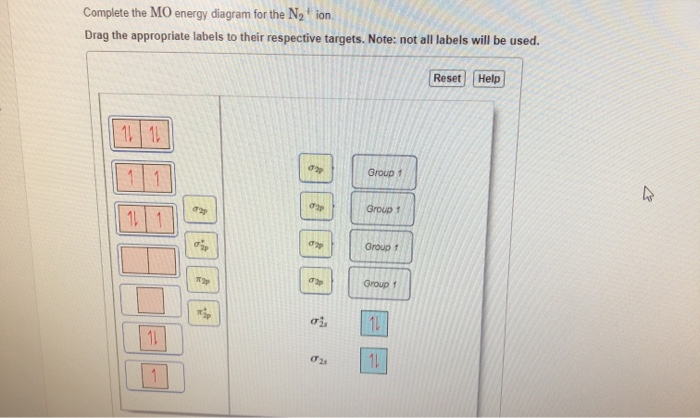
Complete the mo energy diagram for the n2+ ion.
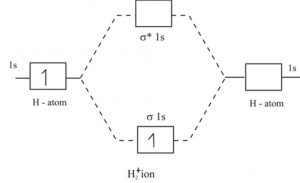
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ... A cation is a positively charged ion with one or fewer electrons than its neutral atom. An anion is a negatively charged ion with one or more electrons than its neutral atom. A polyatomic ion is made up of more than one atom; the whole unit is the ion. 3.36. Titanium lost four electrons to form Ti4+; it has 22 protons and 18 electrons. 3.37 ... 1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is .
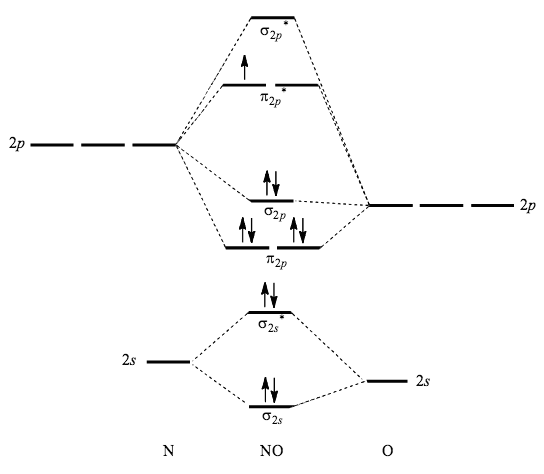
Complete the mo energy diagram for the n2+ ion.. please draw the molecular orbital diagram mo diagram of n2 ion and explain the diagram briefly - Chemistry - TopperLearning.com | es6z6omm Practice Test - MCQs test series for Term 1 Exams ENROLL NOW Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Problem: Complete the MO energy diagram for the N2+ ion. FREE Expert Solution Recall that the bonding MOs are those without an asterisk ( e.g. , σ 1s ) , while the antibonding MOs are those with an asterisk ( e.g. , σ 1s * ) . 1 MOs for Period 2 Homonuclear Diatomic Molecules • Only the valence AOs are considered - one 2s orbital and three 2p orbitals for each atom • When two atoms approach each other: -The 2s orbitals overlap to form two σMOs, bonding (σ2s) and antibonding (σ2s*) (as in H2) -The 2p orbitals directed along the internuclear axis overlap to form two σMOs, bonding (σ2p)
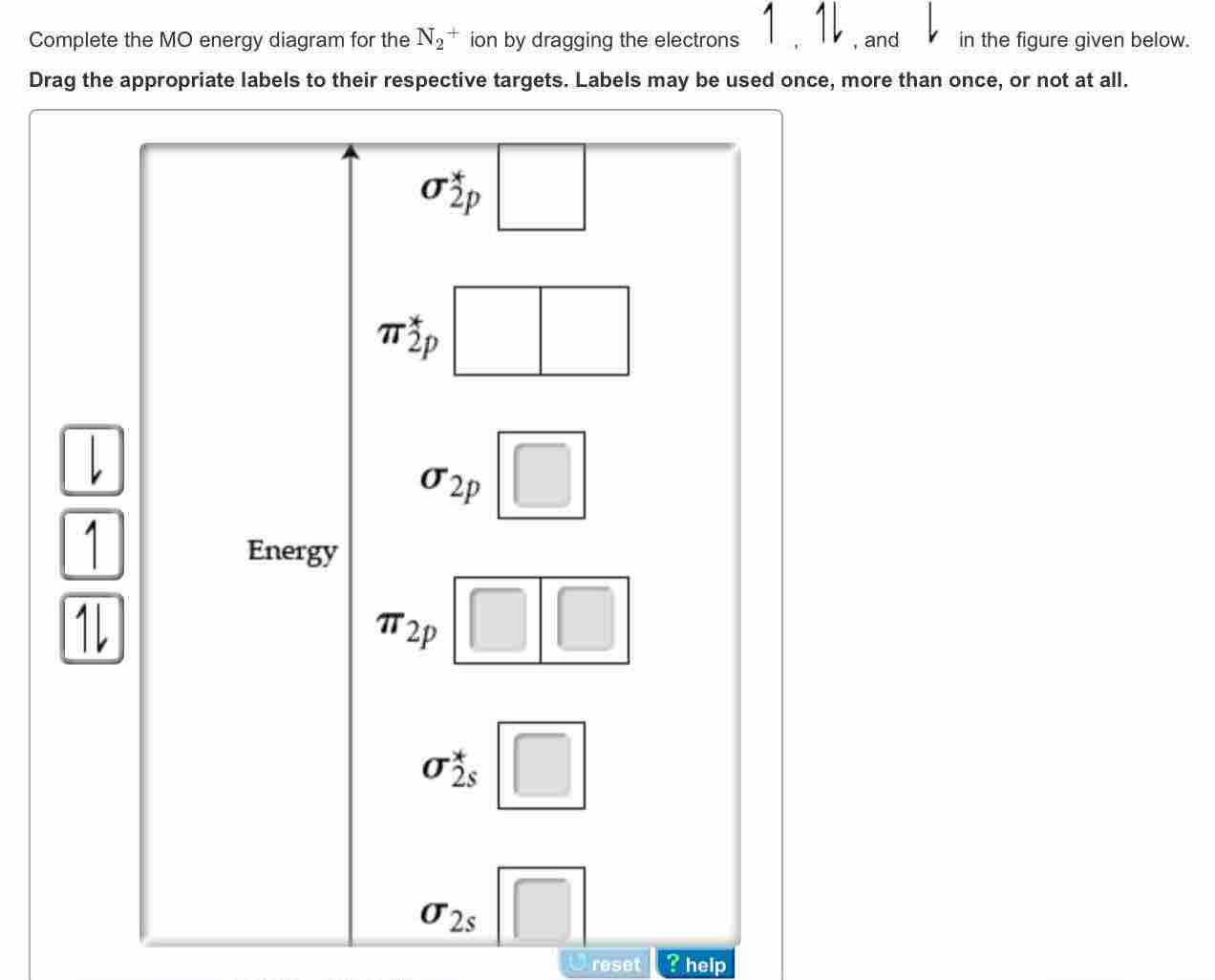
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start... Complete the MO energy diagram for the N2+ ion by dragging the electrons , , and in the figure given below.. Drag the appropriate labels to their respective targets. Labels may be used once, more than once, or not at all. Molecular orbital diagram for ne2 2. 2s. I know ill need a 2p orbital but theres orbital mixing going on so i have 2 choices. Wiki User Answered 2012-03-05 06:41:57. Because According to molecular orbital theory O 2 + has 15 electrons &it has one electron in antibonding orbital. Side by Side Comparison – Homo vs Lumo in Tabular Form 5. C 2 c ... Complete step by step answer: First let us understand the concept of molecular orbital theory. On a very general basis, electrons are not assigned to individual bonds between atoms, but they move under the influence of the nuclei in the whole molecule. Molecular orbital theory is a method for describing the electronic structure of the molecule.
• The following diagram shows the molecular orbital energy level diagrams for the valence electrons in the homonuclear diatomic molecules C 2, N 2 and O 2. Complete the diagram by filling in the remaining valence electrons for each molecule and determining its bond order. Marks 6 Bond order: 2 3 2 M O diagram A has the following orbitals from lowest energy to highest: sigma 2 s, antibonding sigma 2 s, pi 2 p, sigma 2 p, antibonding pi 2 p, and antibonding sigma 2 p. For each of these molecules, identify the proper MO diagram and the number of valence electrons. The 1𝑠 orbital is not shown. Identify the MO diagram for B2. B2 valence e−: Item 2: Part A Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Click here👆to get an answer to your question ️ Draw the molecular orbital energy level diagram of N2 molecules. Solve Study. Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure >> Molecular Orbital Theory
Give The Molecular Orbital Energy Diagram Of N2 And O2 Write The Bond Order Of N2 And O2 Sarthaks Econnect Largest Online Education Community
(Use the energy ordering o Complete an MO energy diagram and determine the bond order for the NO. (Use the energy ordering of N2.) Drag the appropriate labels to their %(1). Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below. Apply molecular orbital theory ...
Item 2: Part A Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below.M.O. diagram for N2+Molecular orbital diagram - Wikipedia
Calculate the energy, in joules, required to excite a hydrogen atom by causing an electronic transition from the n = 1 to the n = 4 principal energy level. Recall that the energy levels of the H atom are given by En = -2.18 × 10^-18 J(1/n2) A) 2.07 × 10-29 J D) 3.27 × 10 …
Formation of molecular orbitals can be determined by LCAO (linear. Continue Reading. Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. so first 2 electrons go in 1s sigma bond. next 2 in 1s sigma anti bond orbital.
Technologies Free Full Text Surface Quality Of Metal Parts Produced By Laser Powder Bed Fusion Ion Polishing In Gas Discharge Plasma Proposal Html
In fact its the perioxide ion. The first photo is straight from a 2006 edition pearson general chemistry textbook and it shows you what the molecular orbital mo diagram for o2 is. Molecular orbital diagram for oxygen gas o2. I actually just covered this question in my gen chem class this week. More molecular orbital diagrams for 02 are provided ...
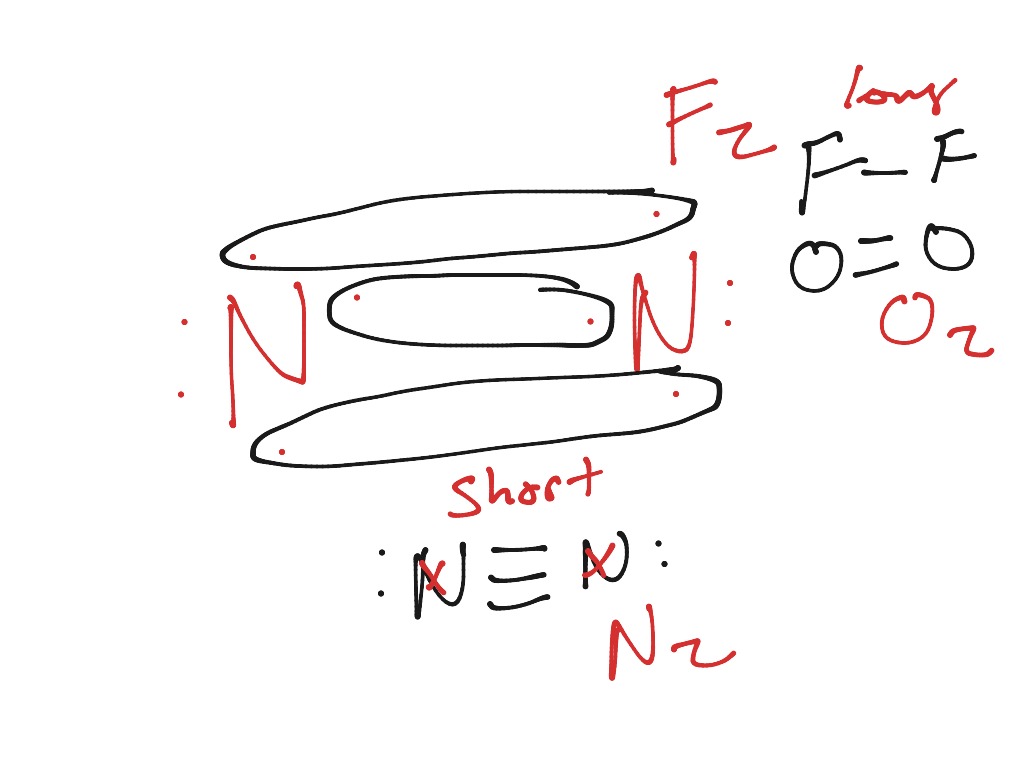
If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion.
The electronic configuration of N2 is KK (σ(2s)) 2 (σ ∗ (2s)) 2 (π(2p x)) 2 (π(2p y)) 2 (σ(2p z)) 2. N b = 8, Na= 2. Bond Order= 3. Bond order value of 3 means that N 2 contains a triple bond. High value of bond order implies that it should have highest bond dissociation energy. Presence of no unpaired electron indicates it to be ...

With The Help Of Molecular Orbital Theory Draw The Molecular Orbital Energy Level Diagram For N 2 Molecule Also Calculate The Bond Order And Predict The Magnetic Behaviour
M.O. Diagram for B2. Postby Rebecca DeShetler 4E » Sun Nov 09, 2014 2:18 am. When you write the M.O. Diagram for B2, this is what you get: This shows two unpaired electrons in π2px and π2pz. However, the Bond Order of B2 = 1/2 (4-2) = 1. Doesn't this mean that B2 has a single bond between the two Boron atoms--therefore they have one sigma bond?
Molecules with Similar Molecular Orbital Diagrams Molecules and ions formed from 2 boron atoms or from 2 carbon atoms have molecular orbitals diagrams of the same sort as N 2. Diatomic molecules made up of two different atoms also have molecular orbital diagrams very similar to that of N 2.When the electronegativity of one atom is lower than the other, the more electronegative atom's orbitals ...
energy molecular orbital (σ*) will be empty (recall the Aufbau Principle). While there are only two ... There would be four electrons to fill into our molecular orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO. What we gain in the bonding sigma MO, we lose in the anti-bonding sigma-star MO.
Other MO diagram constructions can give different electron configurations and bond orders, but should always give no unpaired spins. (d) Use the MO diagram in part (c), changing N for B and O for N. Then, the electron configuration for NO - is: 1σ 2 2σ* 2 1π 4 3σ 2 2π* 2, there are 2 unpaired electrons, and the bond order is 2.
Academia.edu is a platform for academics to share research papers.
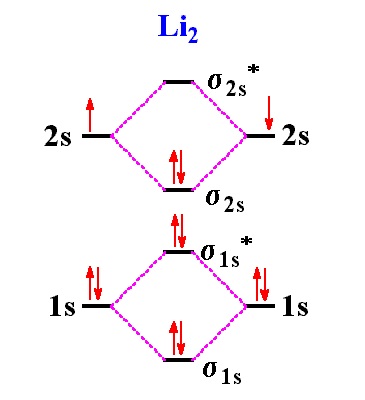
Chemistry Answer | Ion | Chemical Bond. Because lithium is assumed to form 1+ ions in compounds, we do not need to indicate the charge of the metal ion in the compound. Dilithium monoxide would be the name if Li2O were a covalent compound (a compound composed of only nonmetals). 44. CHAPTER 2 45. ATOMS, MOLECULES, AND IONS b. dinitrogen ...
Problem: Draw the molecular orbital diagram for N2- ion, and calculate the bond order. Indicate if it is diamagnetic or paramagnetic. FREE Expert Solution. 99% (88 ratings) FREE Expert Solution. 99% (88 ratings) Problem Details.
I Am Very Confused About Anti Bonding Orbitals How They Form I Mean When Two Atomic Orbitals Produce Bonding Orbital Anti Banding Orbital Please Clear My Basics I Am Really Worried Please Solve
17.1.2017 · The family of 2D transition metal carbides, carbonitrides and nitrides (collectively referred to as MXenes) has expanded rapidly since the discovery of Ti3C2 in 2011. The materials reported so far ...

Complete Orbital Diagrams Boxes With Arrows In Them To Represent The Electron Configuration Of Valence Homeworklib
Draw the MO diagram of CN and fill it with electrons. After that, with the help of the CN Molecular orbital diagram, you can easily predict its properties like bond order, …
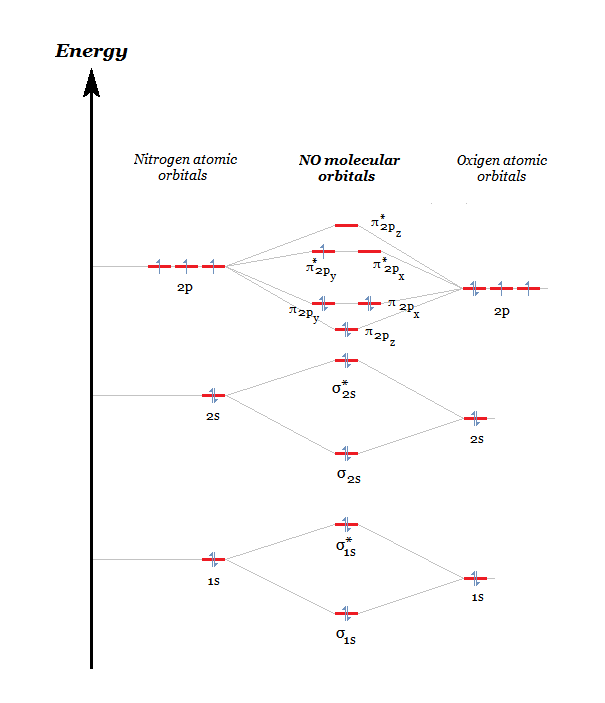
molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial molecular orbital energy-level diagram for the HF molecule.
15.11.2021 · Mo and C powders were previously mechanically activated by milling [148,149]. Yang et al. synthesized Mo 2 C using the MSS method. Firstly, Mo and C powders were mechanically activated under an argon atmosphere using a high-energy planetary mill (10 mm hardened steel ball, ball-to-powder ratio: 5:1, rotational speed 1200 rpm).
Draw the molecular orbital diagram for n2 ion and calculate the bond order. Here is the full molecular orbital diagram for n2. The 2px orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital in the xz plane. They werent drawn that way on this diagram but they should be.
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)).Fill from the bottom up, with 9 valence electrons total.Bonding Order is 2.5, so it is unstable, ...
1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is .
A cation is a positively charged ion with one or fewer electrons than its neutral atom. An anion is a negatively charged ion with one or more electrons than its neutral atom. A polyatomic ion is made up of more than one atom; the whole unit is the ion. 3.36. Titanium lost four electrons to form Ti4+; it has 22 protons and 18 electrons. 3.37 ...

Give The Molecular Orbital Energy Diagram Of A N2 And B O2 Calculate The Respective Bond Order Write The Magnetic Nature Of N2 And O2 Molecules
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...
Microbial Inactivation And Quality Changes In Orange Juice Treated By High Voltage Atmospheric Cold Plasma Springerlink

In The Molecular Orbital Diagram For The Molecular Ion N 2 The Number Of Electrons In The S2p Molecular Orbital Is
Give The Molecular Orbital Energy Diagram Of N2 And O2 Write The Bond Order Of N2 And O2 Sarthaks Econnect Largest Online Education Community
Draw The Molecular Orbital Diagram Of N2 Also Find Its Bond Order And Magnetic Character Chemistry Topperlearning Com 4s4p942zz














0 Response to "40 complete the mo energy diagram for the n2+ ion."
Post a Comment