40 fe3+ orbital diagram
Re: Electron Configuration for Fe 3+. When I work this out, I first write out the structure for the ground state configuration which would be [Ar] 3d^6 4s^2 and then I just take electrons out in the order that is written so Fe3+ would be [Ar]3d^5 since the 4s block has slightly lower energy and they are filled first. Hope this helps! Transcribed image text: Orbital Diagram 30 4 points Fe3+ (this is iron with a positive charge) Determine the orbital diagrams for the following elements AND determine whether the element is paramagnetic or diamagnetic. You will submit a screenshot of your answer for each question. So make sure to do your work on a sheet of paper Drage Drop here or Bewe Orbital Diagram 3 31 4 points Mg ...
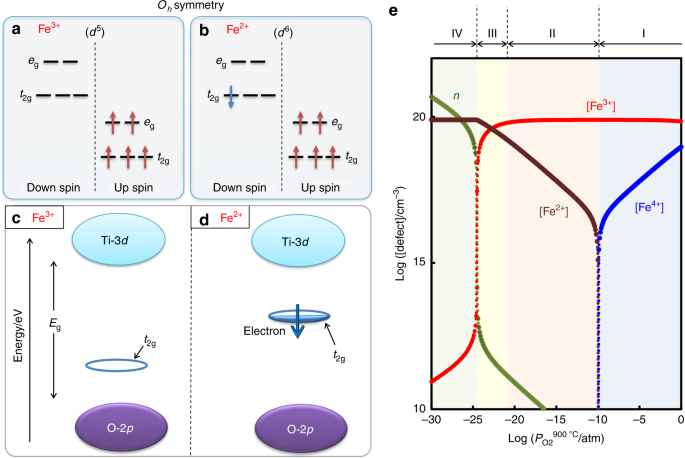
Molecular orbital diagram for the Fe3*Mn2*O,o clus-ter in the (a) ferromagnetic and (b) antiferromagnetic configu-rations. Orbitals indicated with a dashed line are unoccupied. Note that the orbital energies correspond to "orbital electronega-tivities" (Slater, 1974). The energy differences between orbitals

Fe3+ orbital diagram
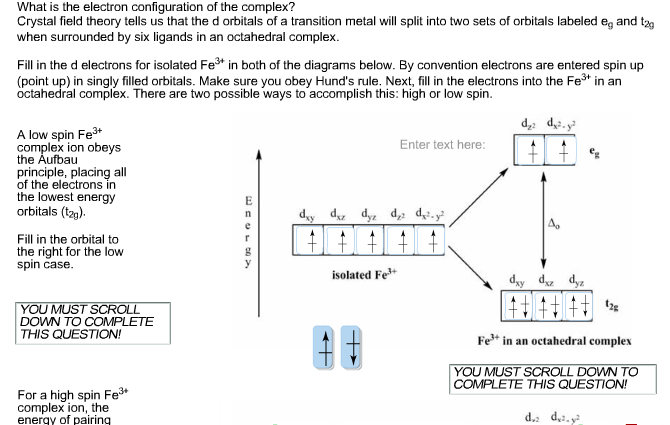
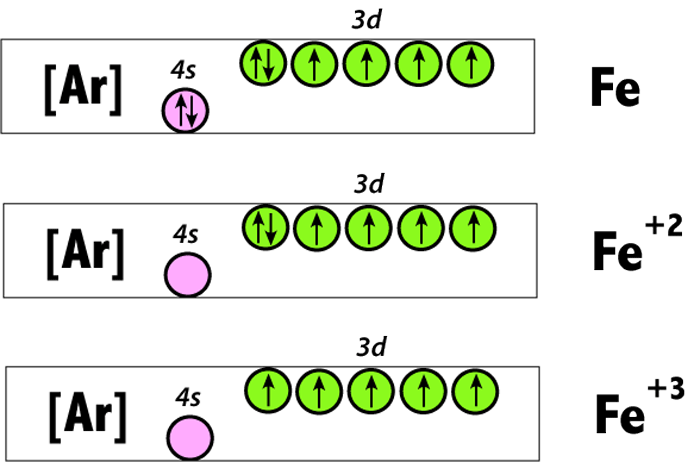
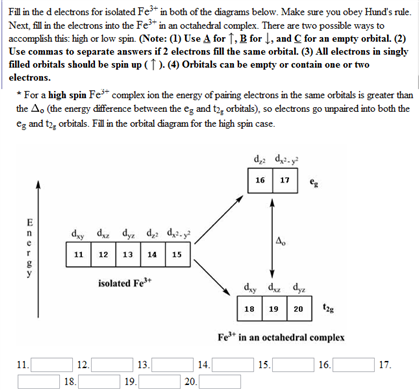
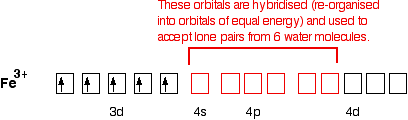
From the periodic table, iron has atomic number 26 , meaning that there are 26 electrons in each ground state iron atom. 2⋅5=10 electron in each d orbital, and so on so forth. Electron orbitals fill according to the Aufbau (Build-up) Principle. Why Fe2+ is easily oxidized to Fe3+? This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
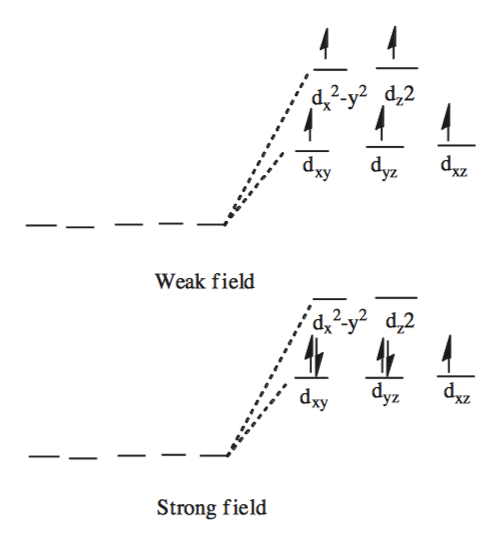
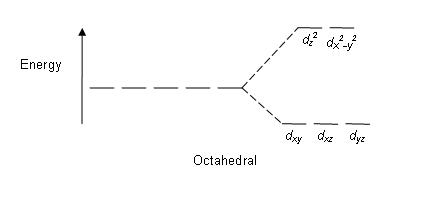
Fe3+ orbital diagram. A qualitative molecular orbital diagram for ferrocene (D 5d) FeII Fe SALC's p p a 2u * e 1u * x e 1u z y a e 2g * e 2g, u a 2u, e 1u a 1g * u a 1g e 1g * LUMO a 1g, e 1g, e 2g e 2g a 1g HOMO dyz dxz e 1g e 1g e 1u e 1g, e 1u a 1g a 2u a 1g, a 2u • Due to a difference in energies the lowest energy a 1g molecular orbital is mainly Fe3+ = Ar, 3d5 which is the stable configuration of Argon+ a half filled d sublevel and a completely empty s sublevel. The half filled d sublevel has one electron in each orbital which is more stable than the d6 which has one orbital filled and four half filled. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... From the periodic table, iron has atomic number 26 , meaning that there are 26 electrons in each ground state iron atom. 2⋅5=10 electron in each d orbital, and so on so forth. Electron orbitals fill according to the Aufbau (Build-up) Principle. Why Fe2+ is easily oxidized to Fe3+?

Electronic Structure Orbital Symmetry Transformation Charge Transfer And Valence State Studies On Fe3 Substituted Cacu3ti4o12 Quadruple Perovskites Using X Ray Photoelectron Spectroscopy Sciencedirect
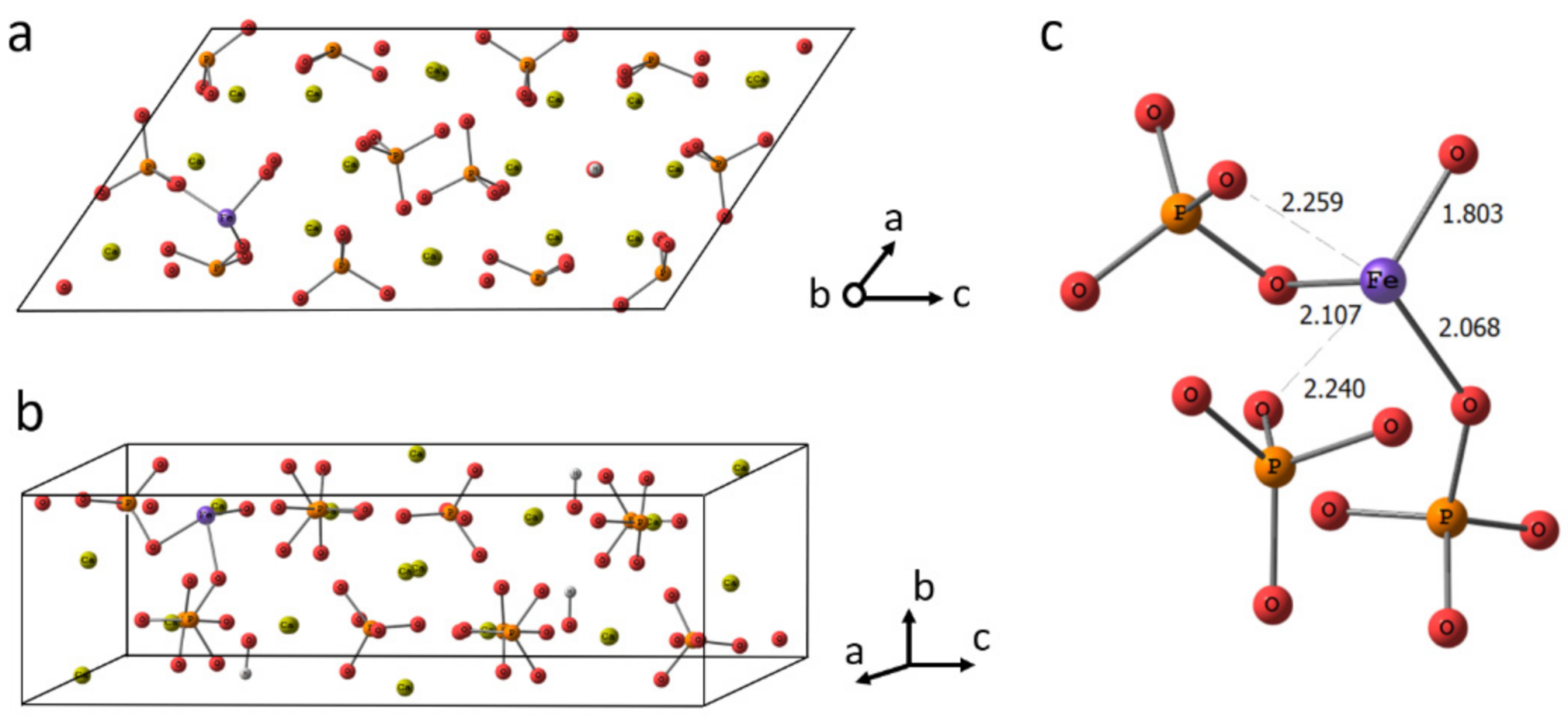
Nanomaterials Free Full Text Mesoporous Iron Iii Doped Hydroxyapatite Nanopowders Obtained Via Iron Oxalate Html

Diketahui Atom Besi Mempunyai Nomor Atom 26 Tuliskan Konfigurasi Elektron Atom Besi Ada Berapa Brainly Co Id

1 Tuliskan Bentuk Konfigurasi Elektron Dan Diagram Elektron Dari Atom Dan Ion Berikut A 27c0c 20 Brainly Co Id

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2

Draw Orbital Box Diagrams For Fe 2 Fe 3 Zn And Zn 2 Tell Which Is Paramagnetic Paramagnetic Means That It Has Unpaired Electrons This Can Only Be Seen With Box Diagrams Study Com
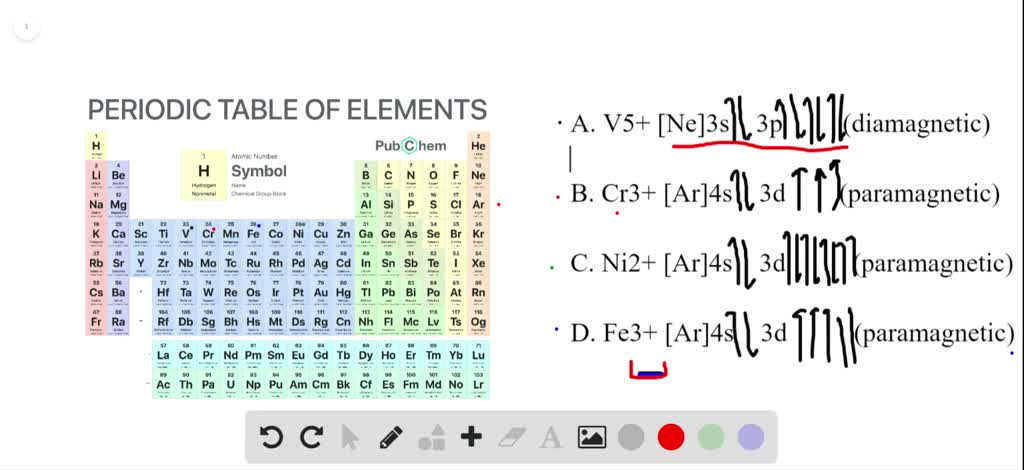
Solved Write Out The Electron Configuration For The Uncharged Fe Atoms And The Fe2 And Fe3 Ions Also Write Out The Orbital Diagram To Show The Unpaired Electrons Is Each Species Paramagnetic Or

Diketahui Nomor Atom Fe 26 Maka Konfigurasi Elektron Ion Fe 3 Baca Fe Pangkat 3 Adalah Brainly Co Id

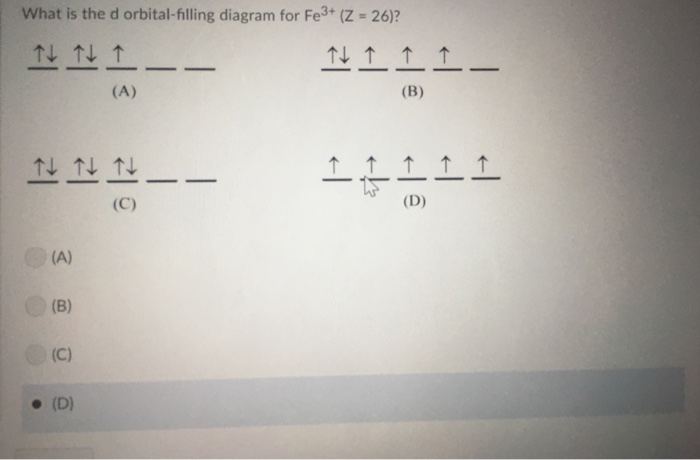










0 Response to "40 fe3+ orbital diagram"
Post a Comment