40 molecular orbital diagram for b2
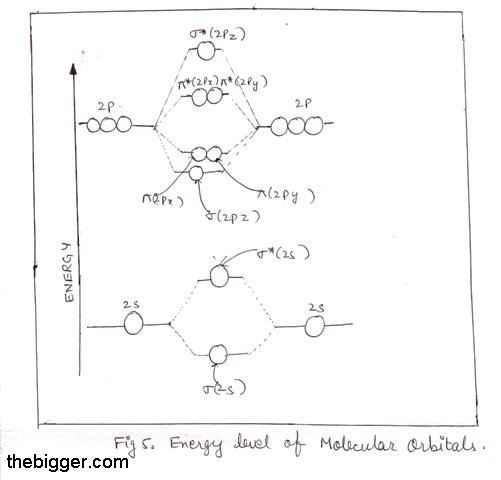
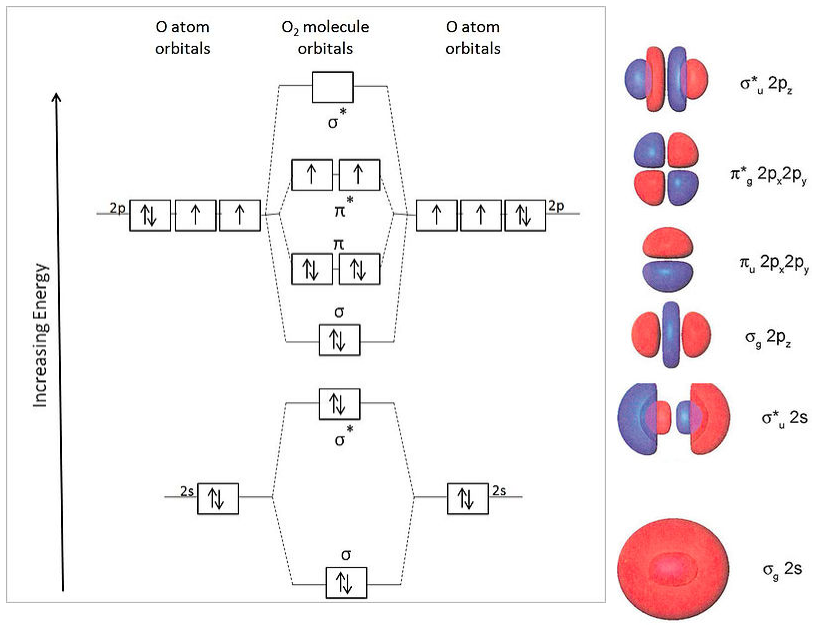
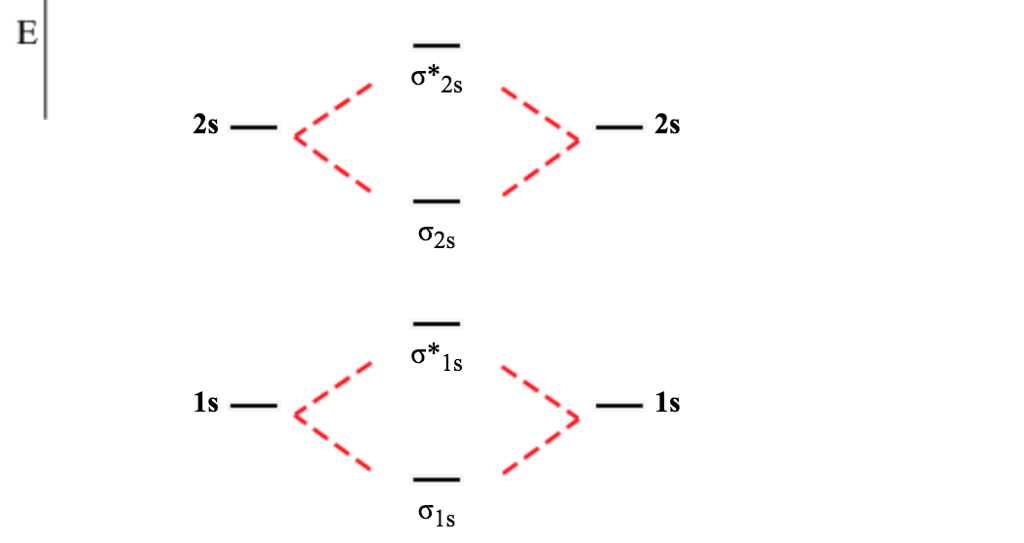
Molecular orbital diagram of b2. Give bond order and predict whether they are diamagnetic or paramagnetic for all the molecules above question 1 3. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of ... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
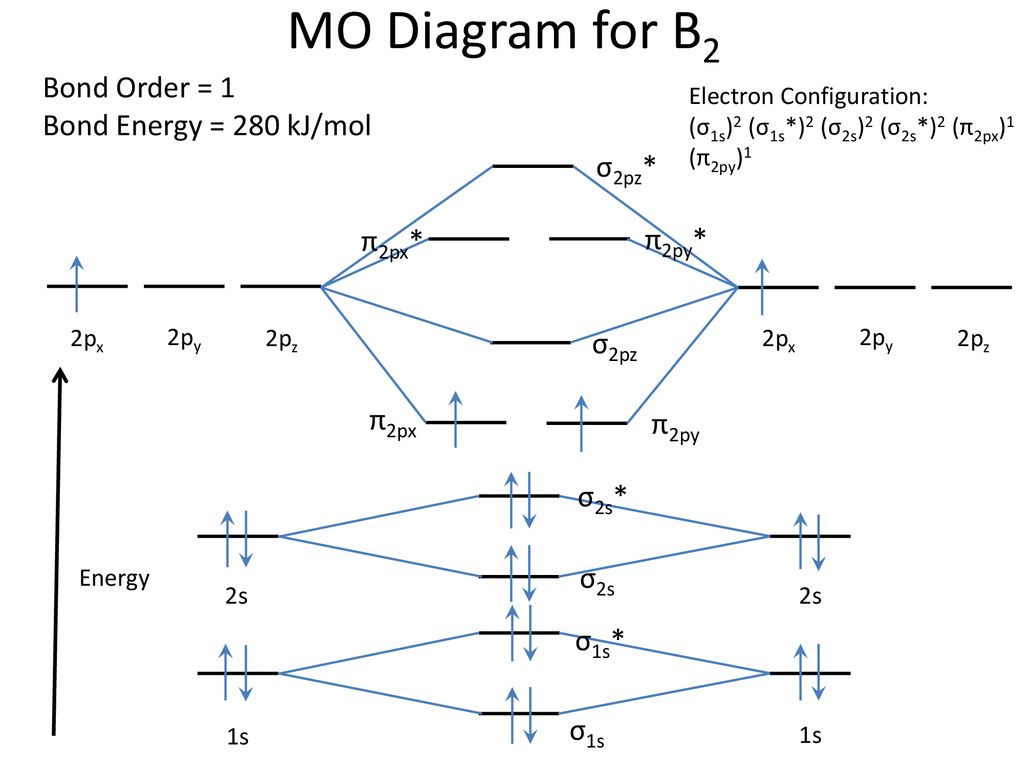
The molecular orbital diagram for B 2 molecule is as follows: We know that bond order is the difference between the number of bonds and the antibonds. Now, we have to calculate the bond order of B 2 molecule using the formula as follows: Bond order = 1 2 ( Number of electrons in BMO) − ( Number of electrons in ABMO) From the diagram, we can ...

Molecular orbital diagram for b2
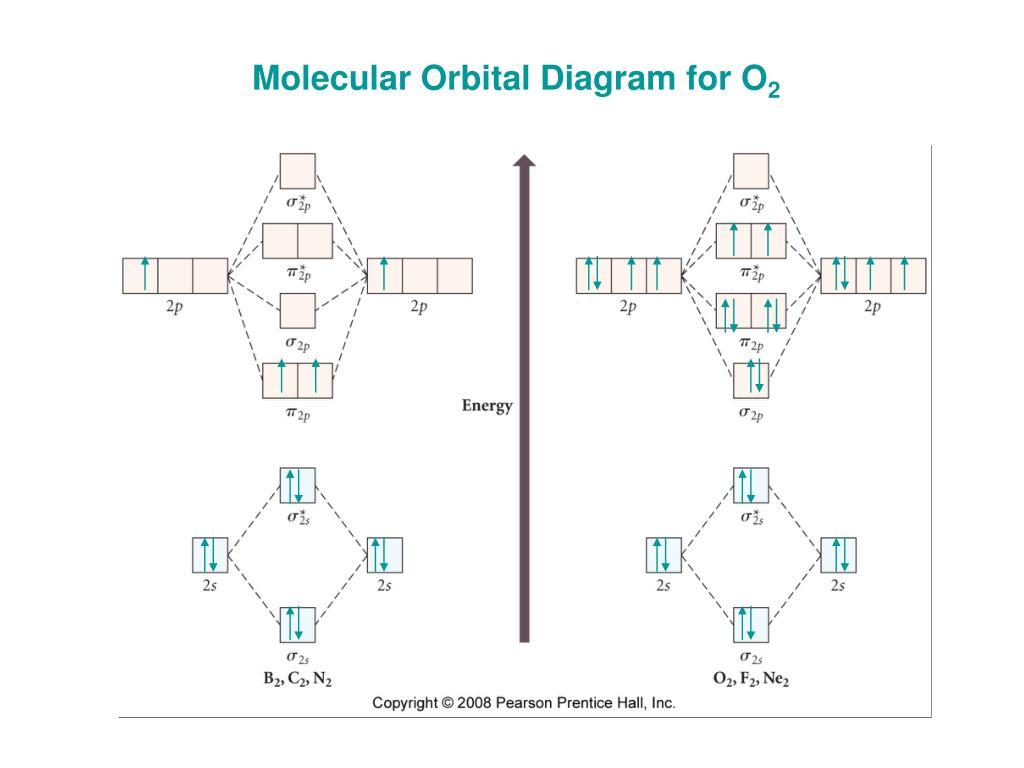
B2 2 molecular orbital diagram. Molecular orbital diagrams of diatomic molecules. Bonding order is 2 and it is diamagnetic. Now for filling the diagram. This video shows the end of the be2 molecule mo diagram and explains pi orbitals paramagnetism and the mo diagrams for b2. The bond order of 32 and the humo appear correct to me as well as how ... From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ... Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
Molecular orbital diagram for b2. Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11. Molecular orbital diagram for beryllium dimer be2 fill from the bottom up with 4 electrons total. The molecular orbital mo theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms. Electrons would be in a bonding orbital we would predict the li2 molecule to be. Molecular orbital diagram for b2. By drawing molecular orbital diagrams for b2 c2 n2 o2 and f2 predict which of these homonuclear diatomic molecules are magnetic. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. Molecular Orbital (MO) Theory helps us to explain and understand certain Part B - Molecular Orbital Energy Diagrams & Bond Order . + and Be2.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic ...
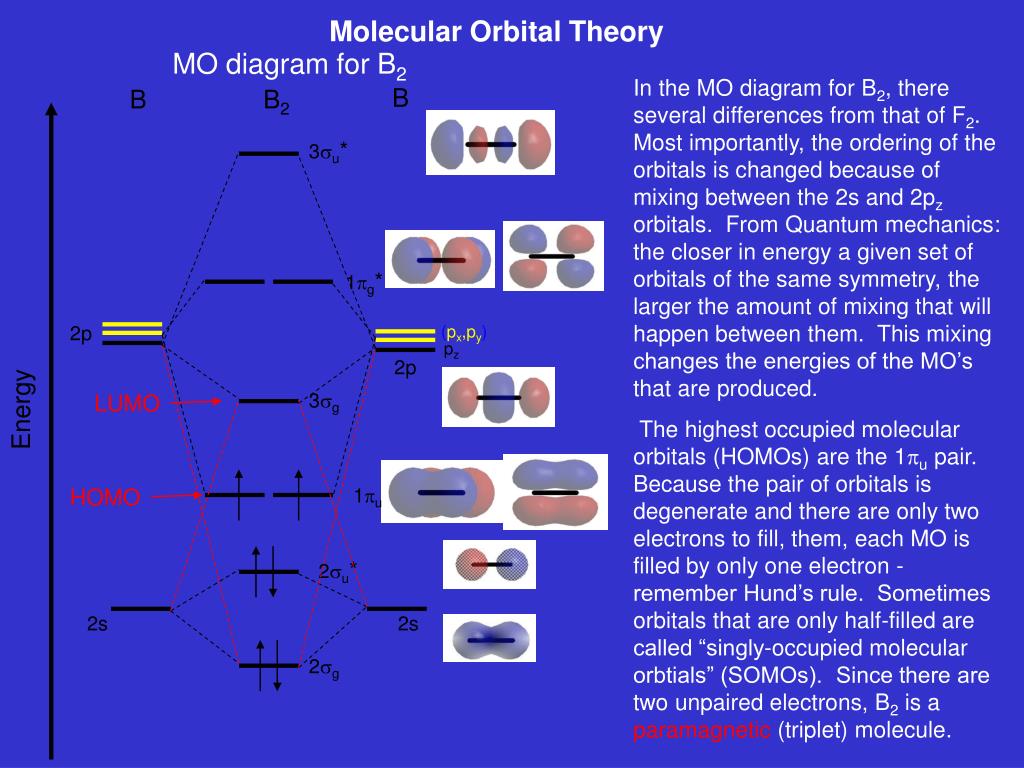
The resulting diagram looks (in approximation) like this: If you now fill the three valence electrons of each boron into the diagram according to Hund's rule, you will see that each π orbital will get one electron. This results in a triplet ground state. The finished valence molecular orbital diagram is pictured below. The output starts with a listing of the orbital symmetries of all orbitals of the system. In this particular example, the highest occupied molecular orbital (HOMO) is orbital No. 8 and belongs to the B 2 irreducible representation (antisymmetric with respect to the principal C 2 axis). Following this information, the orbital energies of all orbitals of the system are given. The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. Each sp 1 hybrid orbital has s-character and The molecular orbital structure of ethylene: In ethene molecule, each carbon atom undergoes sp 2 hybridisation. Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown.
B2 molecular orbital diagram. Since bond order is zero be 2 molecule does not exist. This was on a quiz and i somehow got the bond order and the lumo indicated wrong. I also calculated the bond order of this molecule to be 32. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. 4 draw the molecular orbital diagram ... the valence molecular orbital diagram for the anion B2- is given. Which of the following options correctly interpret this diagram? - B2- has a shorter bond than B2-The molecular orbital bond order is equal to 3/2.-B2- is paramagnetic. B2 molecular orbital diagram. This also causes a large jump in energy in the 2p σ orbital. For example when two hydrogen atoms bond a σ1s bonding molecular orbital is formed as well as a σ1s antibonding molecular orbital. Valence bond model vs. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both ... Molecular Orbital Diagram Be2. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown. From the above MO diagram we can see that number of elctrons in the bonding and ...
This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the MO diagrams for B2.
"BO" = 1/2 Boron atom is atomic number 5 in the periodic table, so it has five electrons. Thus, B_2 carries ten total electrons. The atomic orbitals each boron contributes consists of the 1s, 2s, and 2p. The ns orbitals combine to give a portion of the molecular orbital (MO) diagram like this: where sigma^"*" indicates an antibonding sigma (sigma) MO, and sigma is the bonding MO.
Molecular orbital diagram for b2. The organic chemistry tutor 569143 views. Additionally i labelled the humo as pi2py and the lumo as sigma2px. Molecular orbital diagrams of diatomic molecules. Molecular orbitals of the second energy level. F2 o2 and f2 o2 and b2 o2. Hybridization of atomic orbitals sigma and pi bonds sp sp2 sp3 organic ...
Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape.. There are two types of MO diagrams:. Recall that the bonding MOs are those without an asterisk (e.g., σ 1s), while the antibonding MOs are those with an asterisk (e.g., σ 1s *).

3 Which Of The Following Species Have Both O And It Bond According To Molecular Orbital Theory 1 Nz 2 B2 3 Cz Canly Tband 4 All Of These
Re: M.O. Diagram for B2 Post by Chem_Mod » Tue Nov 11, 2014 11:21 pm As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond.
A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals method in particular. However the bond order of b2 124 2 1. This means there are 5 valence electrons.

Part A By Drawing Molecular Orbital Diagrams For B2 C2 N2 O2 And F2 Predict Which Of These Brainly Com
Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero ; Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired ...
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...

Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic N22 B22 Homeworklib
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Molecular orbital diagram for b2. This interaction introduces an element of s p mixing or hybridization into the molecular orbital theory. When p s mixing is allowed the energies of the σ2p and π2p orbitals are reversed. The two electrons from the b 2p orbitals now occupy separate degenerate π2p molecular orbitals and thus have parallel spins.
Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ...
B2 2 molecular orbital diagram. Molecular orbital diagrams of diatomic molecules. Bonding order is 2 and it is diamagnetic. Now for filling the diagram. This video shows the end of the be2 molecule mo diagram and explains pi orbitals paramagnetism and the mo diagrams for b2. The bond order of 32 and the humo appear correct to me as well as how ...
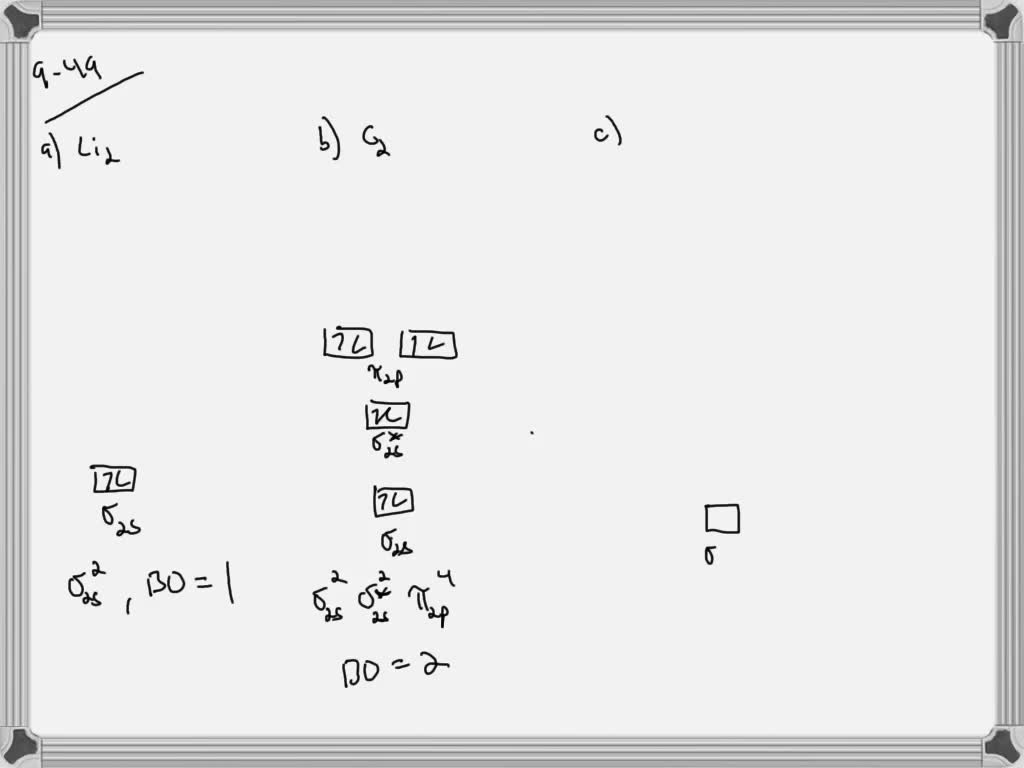
Solved Draw The Molecular Orbital Diagrams For B2 C2 And N2 Please Use It To Predict The Bond Order For B2 B2 B2 C2 C2 C2 N2 N2 And N2 Which















0 Response to "40 molecular orbital diagram for b2"
Post a Comment