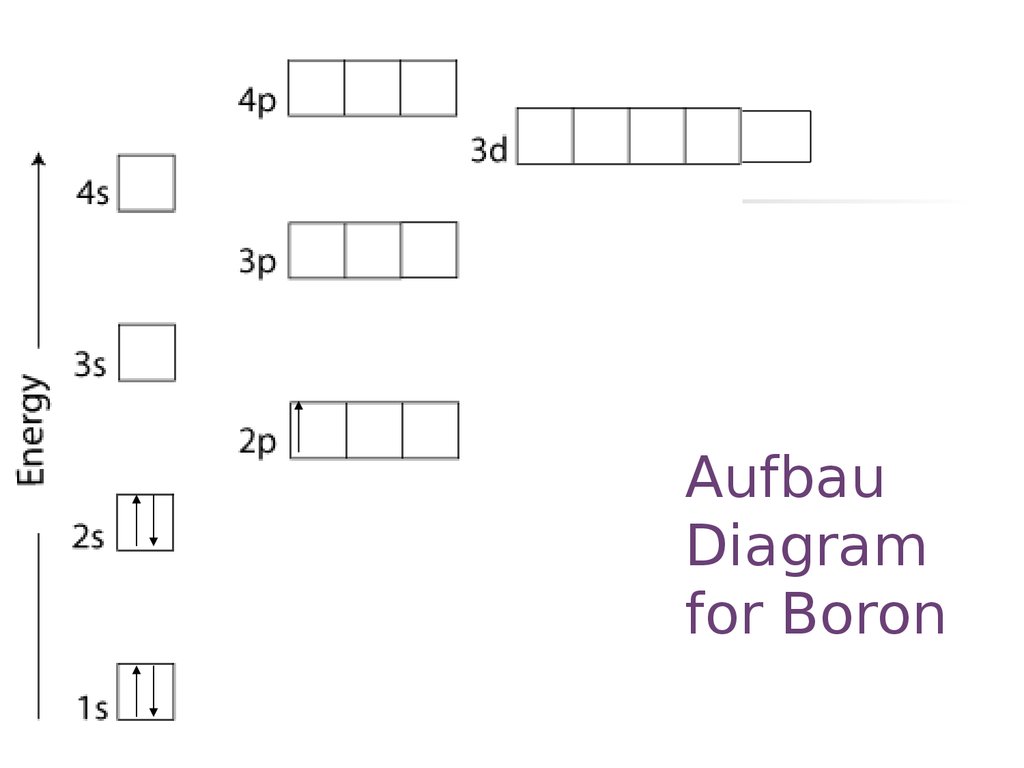
40 orbital diagram of boron
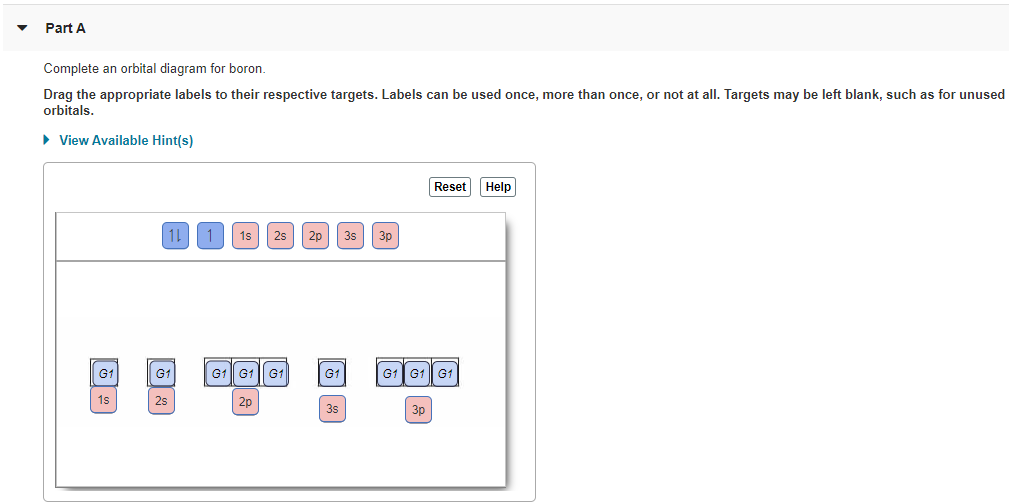
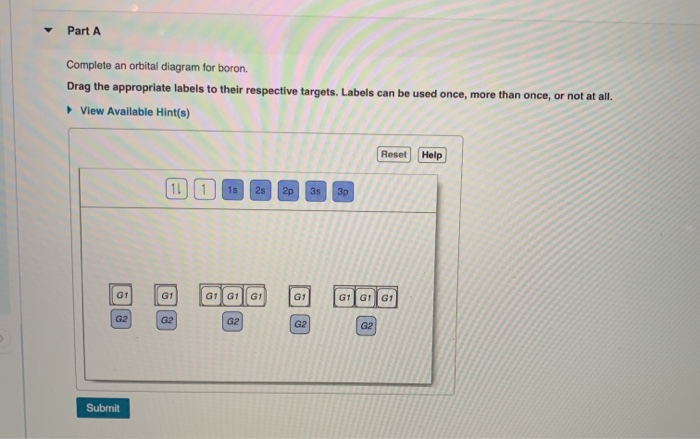
The orbital diagram has nine boxes with two arrows in the first seven and single arrows in the last two Write the electron configuration and draw the orbital notation for atoms of oxygen and sulfur. You've reached the end of your free preview. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p Boron. ** the second 2p ... The molecular orbital diagrams of , and are drawn in the attached image. There is no unpaired electron present in the MO diagram of and all the electrons are paired up so it is diamagnetic in nature. There is one unpaired electron present in the MO diagram of and therefore it is paramagnetic in nature.
Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.The chemical symbol for Boron is B. Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. There are over 100 different borate minerals, but the most common are: borax, kernite, ulexite etc. Natural boron consists primarily of two ...
Orbital diagram of boron
Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic. asked Dec 17, 2020 in Chemical Bonding by Panna01 ( 47.2k points) chemical bonding Orbital diagrams are photographic descriptions of the electrons in one atom. Three rules are helpful in developing orbital diagrams. ... So for the element of BORON, you already know the the atom number tells you the variety of electrons. That way there are 5 electrons in an boron atom. Use the orbital-filling diagram to show the electron configuration of aluminum, AlAl. Electron configuration s are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron configuration of boron.
Orbital diagram of boron. The molecular orbital diagram of BeCl2 will be drawn by combining atomic orbitals of beryllium atom and group orbitals of chlorine atom having similar energy and symmetry around a molecular axis. The 3s group orbitals of chlorine atom will remain non-bonding because their energy is very low as compared to the 2s and 2p atomic orbitals of ... Boron has atomic number 5. B is the fifth element of the periodic table having total 5 electrons. Two electrons will occupy the lowest energy subshell 1s then remaining 2 electrons will occupy 2s subshell and 1 electron in 2p subshell. so the electronic configuration of Boron (B) is 1s 2 2s 2 2p 1 . Feb 23, 2016 — The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹ ... The orbital filling diagram of boron. This gives us an orbital filling diagram of. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. Use this tool to generate the electron configuration of arsenic as. Labels can be used once more than once or not at all.
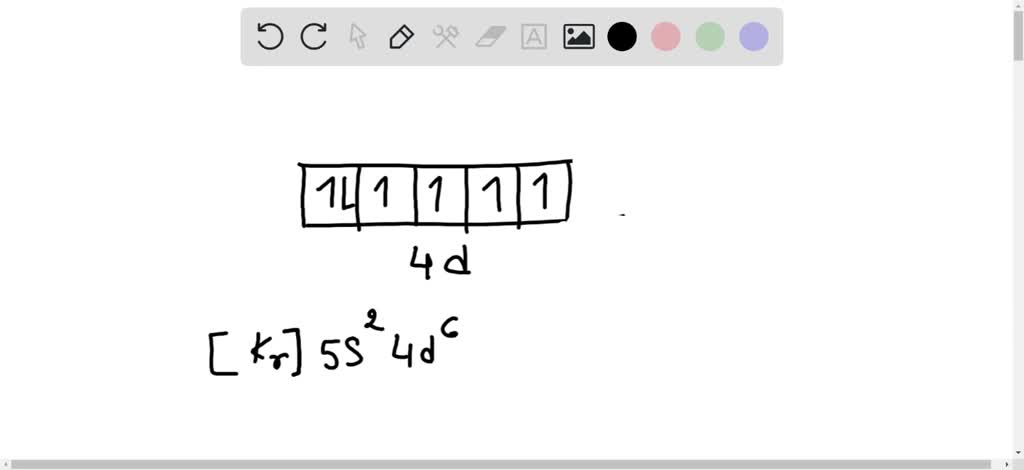
The Molecular Orbital Theory (TOM) is a mathematical model developed to explain the physicochemical properties of molecules, such as the absorption and emission of radiation, electrical conductivity, as well as the electronic nature of their bonds. This considers, unlike the valence bond theory (VTE), that the electrons are delocalized throughout the molecule, without being probabilistically ... Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital.Oct 24, 2016 · Uploaded by Wayne Breslyn Molecular Orbital Diagram of N 2 Chapter 9 Section 6 Molecular Orbital Diagram for B, C, N, O, F and Ne (2) - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Scribd is the world's largest social reading and publishing site.. MO diagram of Ne2 molecule: Ne - 10 - 1 s2 2 s2 2 p6 σ 1s2 σ 2s2 π 2px2 π 2py2 σ 2pz2 Ne... Orbital diagrams are pictorial descriptions of the electron in an atom. Three rules are beneficial in developing orbital diagrams. ... So for the element of BORON, you currently know the the atom number speak you the variety of electrons. That means there room 5 electrons in one boron atom.
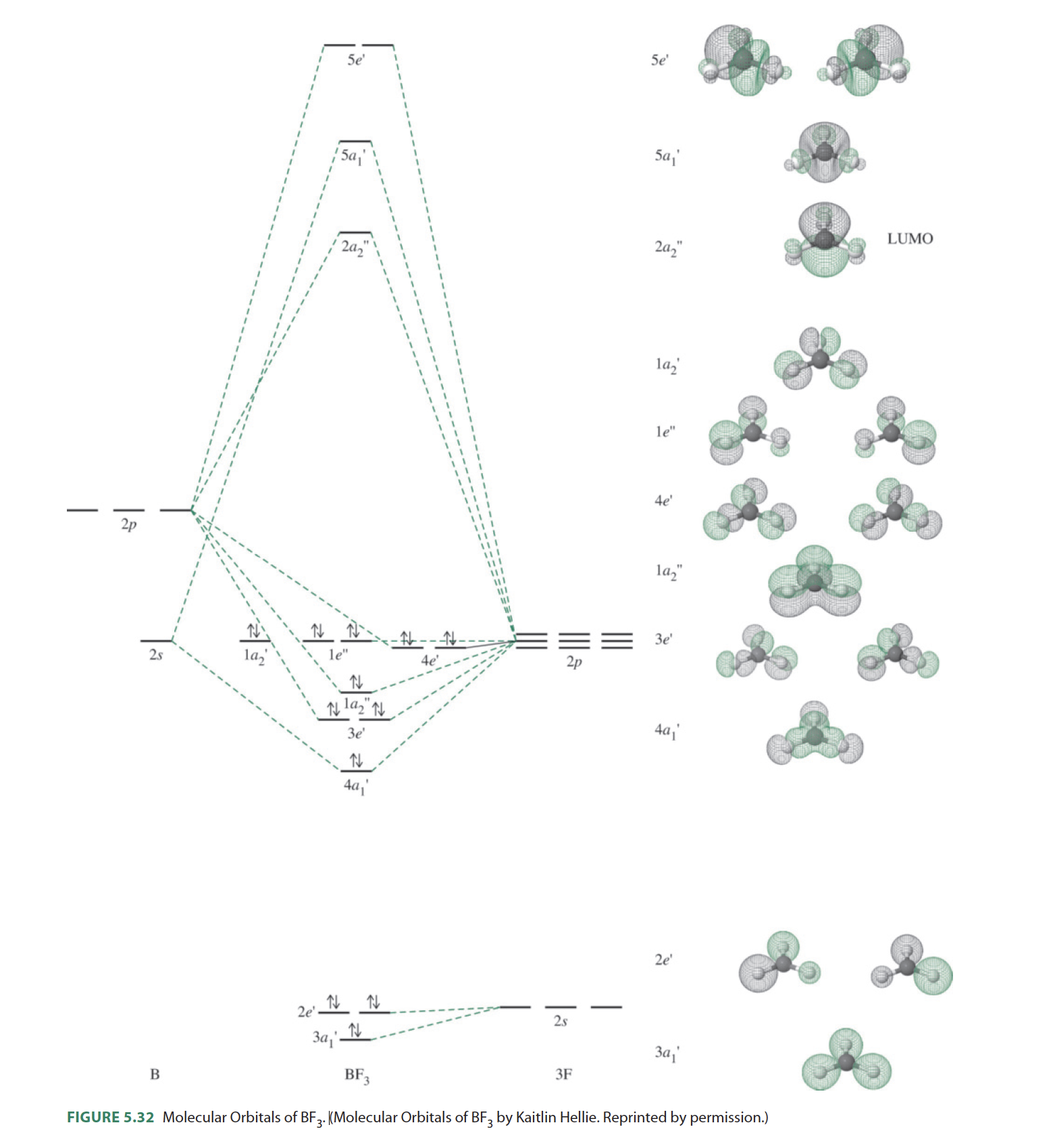
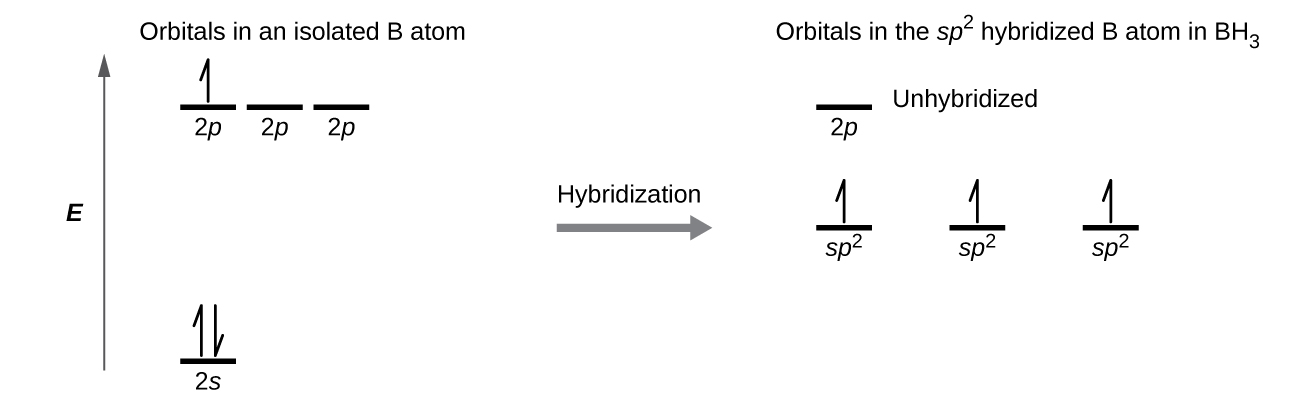
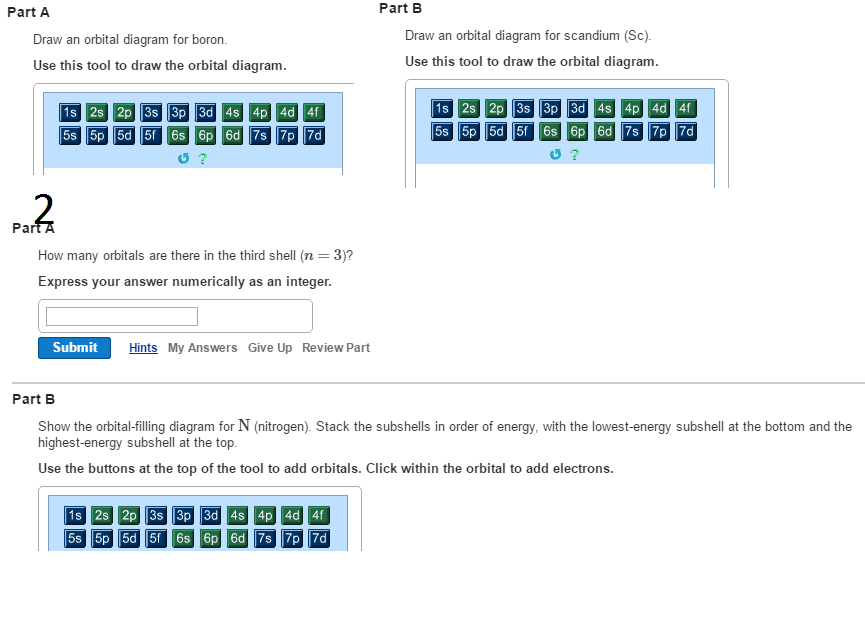
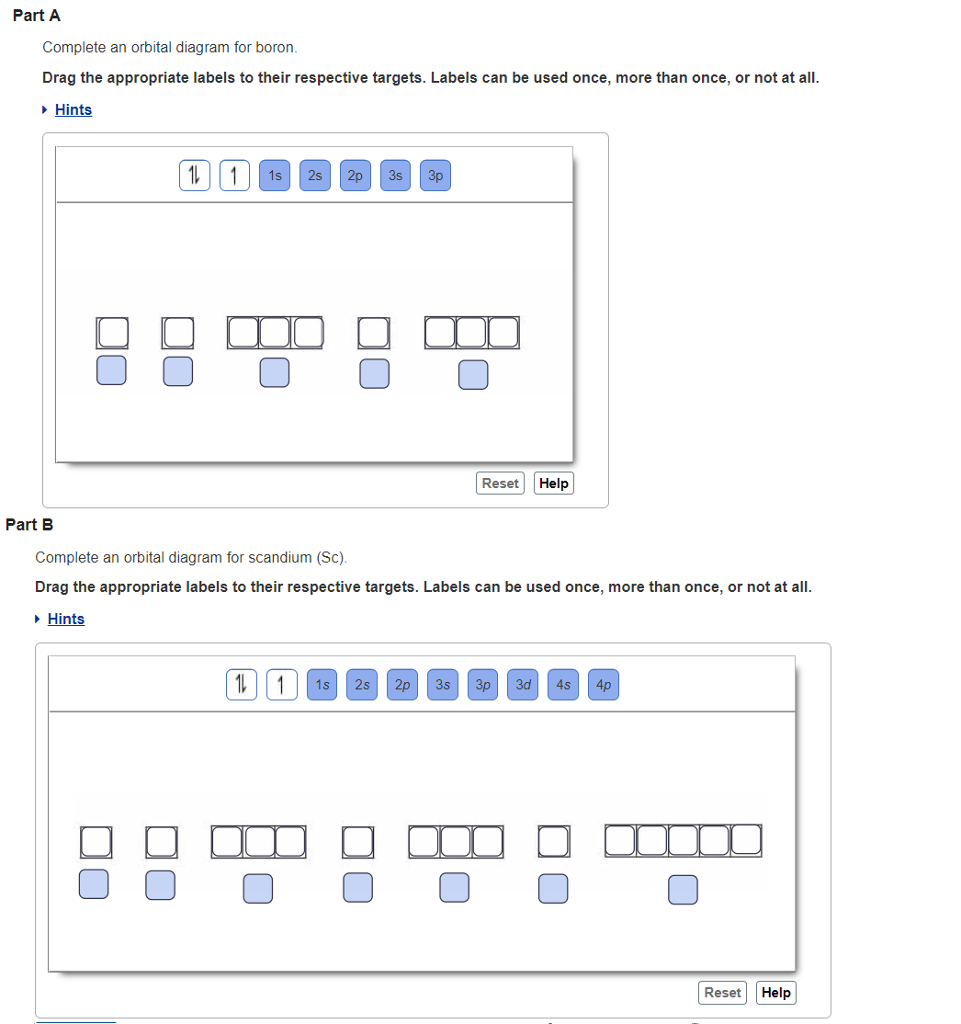
You can complete the orbital diagrams of Boron (B) and Scandium (Sc) by referring to the periodic table, locating the position of each element in it, and ...Sep 16, 2020 Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Therefore the B electron configuration will be 1s 2 2s 2 2p 1. Furthermore, Which is the electron configuration for boron quizlet?, The electron configuration of boron is 1s (2) 2s (2) 2p (1). BF3 is SP2 hybridization. For this molecule, It is SP2 because one π (pi) bond is required for the double bond between the Boron and only three σ bonds are formed per Boron atom. The atomic S - orbitals and P - orbitals in Boron outer shell mix to form three equivalent SP2 hybrid orbitals. The characteristic of the element Boron is that it is a low abundance inside the earth's crust. How Many Valence Electrons Does Boron Have. Certain properties of Boron Electron Configuration are that it is in the form of powder which is brown in color and it is used in boron filaments.
Its FREE youre just one step away. What is the electron configuration for boron. 119 Zeilen This decides the electron capacity of the shells. Carbon He2s 2 2p 2. An orbital diagram is similar to electron configuration except that instead of indicating the atoms by total numbers each orbital is shown with up and down.

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
Molecular orbital diagram of b2.Give bond order and predict whether they are diamagnetic or paramagnetic for all the molecules above question 1 3. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of ... Molecular Orbital s of the Second Energy Level.
Apr 5, 2017 · 1 answerIf you carefully read the description [modiagram_en.pdf][1] , you can see the description of the \molecule command on page 6:.
Structure And Bonding In Boron Carbide The Invincibility Of Imperfections New Journal Of Chemistry Rsc Publishing Doi 10 1039 B618493f
There are a total of 6 electrons to add to the molecular orbital diagram 3 from boron and 1 from each hydrogen atom. Molecular orbital diagram as a non bonding molecular orbital. Sp hybrid orbitals in beh2 1. Then we can put the molecular orbital diagram together starting with the outside drawing in bonding non bonding and anti bonding mos and ...
Electron Configuration Chart of All Elements (Full Chart) June 10, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.
Jan 27, 2015 · 1 answerBefore we can draw a molecular orbital diagram for B₂, we must find the ... Each boron atom has one 2s and three 2p valence orbitals.
Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ...
Solved What Is The Electron Configuration Abbreviated Configuration And Orbital Filling Diagram For The Element Boron
Boron(B) Electron Configuration with Full Orbital Diagram ... Boron electron configuration is 1s2 2s2 2p1. The period of boron is 2 and it is a p-block element.
Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14:
Boron (atomic number 5) has five electrons. Four electrons fill both the 1s and also 2s orbitals. The 5th electron is included to a 2p orbital, the sublevel next greater in power (Figure 5.9). The electron configuration of boron is: B: 1s22s22p1. Table 5.2 shows the electron configurations of the elements with atomic numbers 1 with 18.
Boron, a chemical element with the symbol B and atomic number 5, is a low-abundance element found in the solar system. ... Valence electrons are the total number of electrons present in the outermost shell of an atom (i.e. in outermost orbital). The valence electrons for a neutral atom is always definite, it cannot be varied (more or less) in ...
What is the orbital diagram for phosphorus? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. ... Boron atoms have 5 electrons and the shell structure is 2.3. The ground state electron configuration of ground state gaseous neutral boron is [He]. 2s2. 2p1 and the term ...
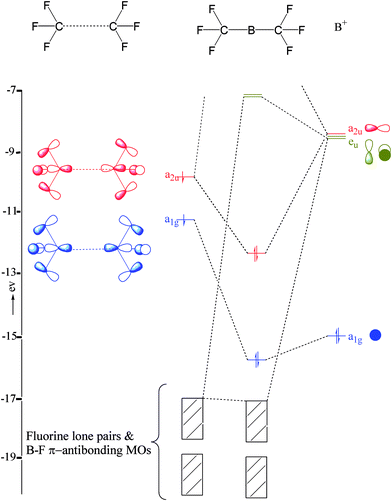
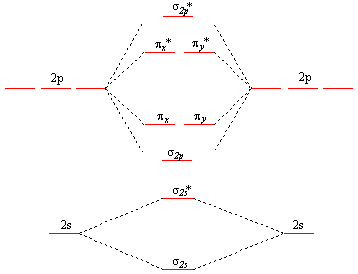
Molecular orbital diagrams give us an idea about the mixing of orbitals in molecules. Let's look into the MO diagram of boron trichloride. The blue color refers to the atomic orbitals of boron, the red color refers to the atomic orbitals of chlorine and the molecular orbital of the molecule is indicated by the purple color.

Show The Orbital Diagrams For The Following Chromium Atom And Chromium Iii Ion Include Only Occupied Orbital S A Cr B Cr 3 Study Com
By Hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Draw an orbital diagram for nitrogen, Z = 7. ১০ ফেব, ২০২১
Orbital diagram of cobalt an orbital diagram is a way of showing where and how many electrons are in an atom of a certain element. The orbital diagram for the atom of cobalt is shown below. 1s2 2s2 2p1 boron 1s2 2s2 2p6 3s2 3p6 4s2 3d1. The Atom Cobalt Has 27 Electrons How Many Energy Levels Will Figure 2 From Methylcobalamin 39 S Full Vs Half ...
Draw And Explain The M O Diagram Of Boron Molecule Sarthaks Econnect Largest Online Education Community
Use orbital filling diagrams to describe the locations of electrons in an atom. ... Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1.
The orbital diagram for boron as shown has the one electron in the 2p orbital. An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. 12 Votes An atom of boron atomic number 5 contains five electrons. 1st s2 2nd s2p6 3rd s2p6d10. Since 1s can only.
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting many compounds such as boric acid, the mineral borax, and the ultra-hard crystal boron carbide.
Lewis Dot Diagram of Boron (B). Description: Hard, brittle, lustrous black semimetal. Exists in the earth's crust at an average proportion of about 10 parts ...
Use the orbital-filling diagram to show the electron configuration of aluminum, AlAl. Electron configuration s are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron configuration of boron.
Orbital diagrams are photographic descriptions of the electrons in one atom. Three rules are helpful in developing orbital diagrams. ... So for the element of BORON, you already know the the atom number tells you the variety of electrons. That way there are 5 electrons in an boron atom.
Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic. asked Dec 17, 2020 in Chemical Bonding by Panna01 ( 47.2k points) chemical bonding
















0 Response to "40 orbital diagram of boron"
Post a Comment