40 what is the basis for exceptions to the aufbau diagram
The aufbau principle, from the German Aufbauprinzip (building-up principle), also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most ... What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the aufbau diagram? A. filled and half-filled energy sublevels are more stable than partially-filled energy sublevels B. electron configurations are only probable C. electron spins are more important than energy levels in determining electron configuration D. some elements have unusual atomic orbitals

What is the basis for exceptions to the aufbau diagram
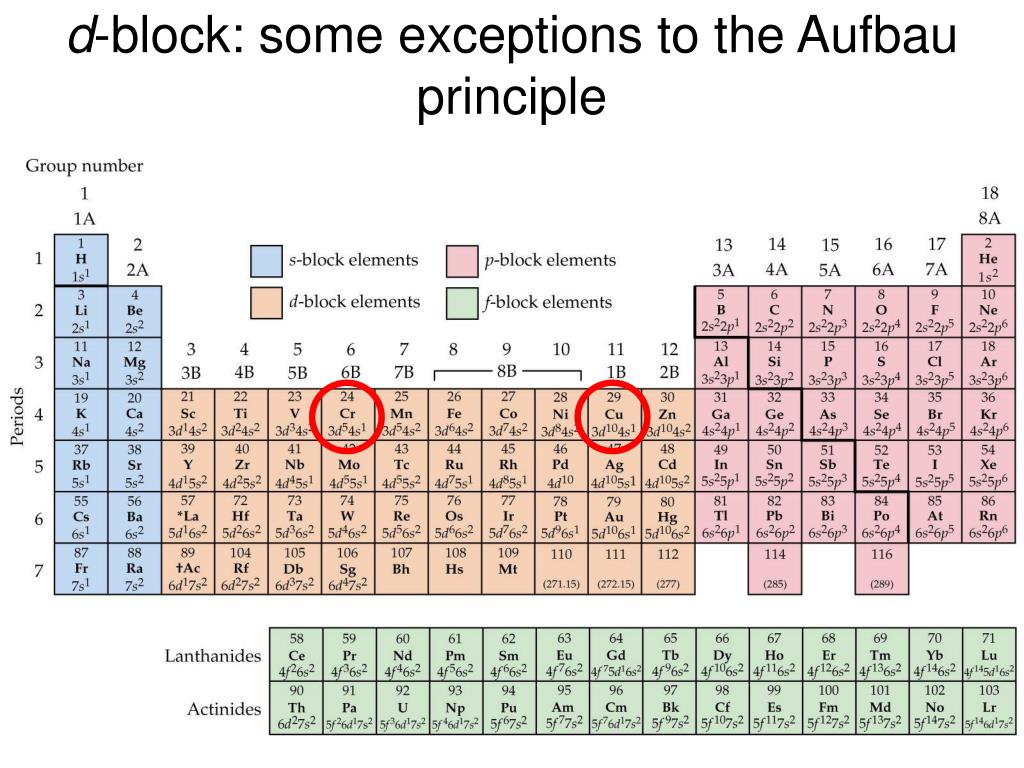
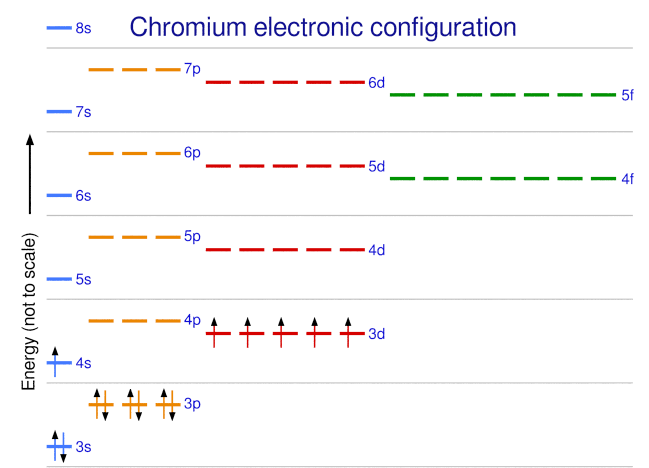
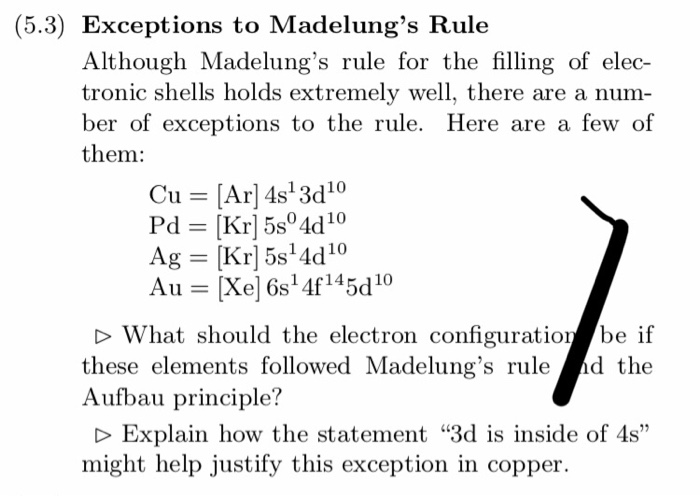
What is the basis for exceptions to the Aufbau diagram?Get the worksheet from here: https://madecalculators.blogspot.com/2020/11/chemistry-more-questions.html Aufbau Principle Exceptions . Like most rules, there are exceptions. Half-filled and completely filled d and f subshells add stability to atoms, so the d and f block elements don't always follow the principle. For example, the predicted Aufbau configuration for Cr is 4s 2 3d 4, but the observed configuration is actually 4s 1 3d 5. This actually ... What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the aufbau diagram. Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell.According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels. Academia.edu is a platform for academics to share research papers. What is the basis for exceptions to Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, ... A diagram illustrating the order in which atomic orbitals are filled is provided below. Here, 'n' refers to the principal quantum number and 'l' is the azimuthal quantum number. The Aufbau principle can be used to understand the location of electrons in an atom and their corresponding energy levels.
What is the basis for exceptions to the Aufbau principle? The Aufbau Principle It states that electrons fill the atomic orbitals of the lowest available energy levels before occupying higher levels. What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels. Aufbau diagram for lithium. The electron configuration of lithium, shown on an Aufbau diagram. The following steps detail how to draw an Aufbau diagram: Determine the number of electrons that the atom has. Fill the s orbital in the first energy level (the 1s orbital) with the first two electrons. We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, master's or pHD, and we will assign you a writer who can satisfactorily meet your professor's expectations.
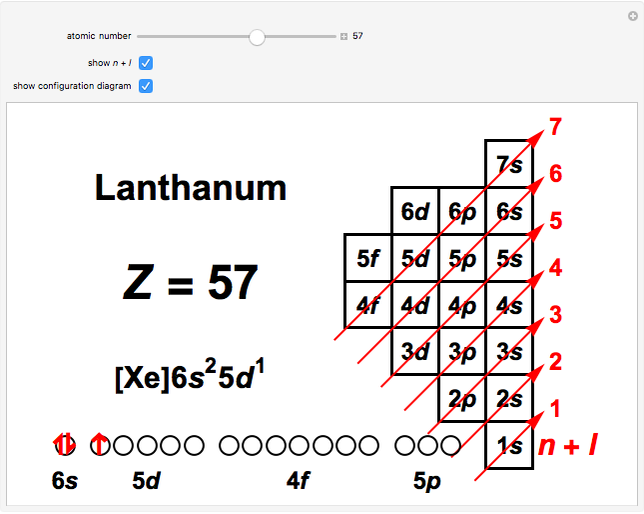
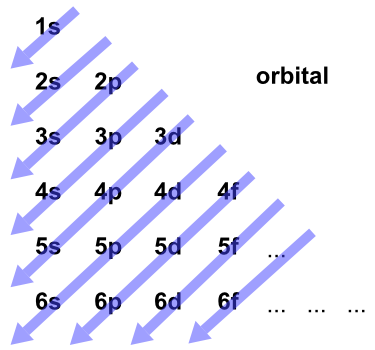
aufbau principle. tendency of electrons to enter orbitals of lowest energy first. electron configuration. ... What is the basis for exceptions to the aufbau diagram? Filled and half-filled energy sublevels are more stable than partially-filled energy sublevels. Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu. The Aufbau Principle: the (n + l) Rule. We've all seen and use the so-called Aufbau Diagram (Figure 1). It is a mnemonic used to remember the order of "filling" of atomic orbitals during the construction of the ground state electron configurations of the elements. The presentation of this diagram is largely disconnected from any physical ... What is the basis for exceptions to the aufbau diagram? Filled and half-filled energy sublevels are more stable than partially-filled energy sublevels. Which scientist developed the quantum mechanical model of the atom? Erwin Schrodinger. The quantum mechanical model of the atom..
Expatica is the international community’s online home away from home. A must-read for English-speaking expatriates and internationals across Europe, Expatica provides a tailored local news service and essential information on living, working, and moving to your country of choice. With in-depth features, Expatica brings the international community closer together.
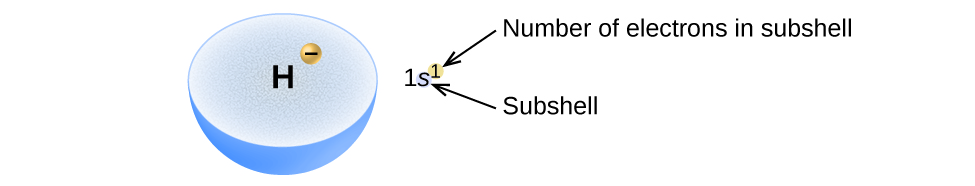
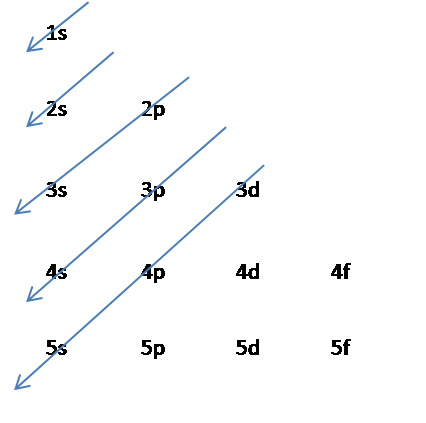
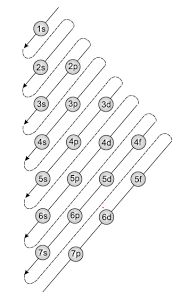
The Aufbau principle is illustrated in the diagram by following each red arrow in order from top to bottom: 1s, 2s, 2p, 3s, etc. Summary The Aufbau principle gives the order of electron filling in an atom.
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels. ...
The Latin alphabet forms the basis of the English alphabet, it is the same alphabet, with the exceptions of J, U, and W. What are the ratings and certificates for Aufbau - 1951?
What is the basis for exceptions to the aufbau diagrams. Other exceptions are copper and silver. In bohrs model of the atom where are the electrons and protons located. Despite the exceptions the aufbau principle is useful in chemistry courses where students discover the fundamental rules about the atomic structure and properties of elements.

The Problem With The Aufbau Principle For Finding Electronic Configurations The Trouble With Using The Aufbau To Find Electronic Configurations
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
34 What Is The Basis For Exceptions To The Aufbau Diagram. Academia.edu is a platform for academics to share research papers. This chemist's explanation of electrolysis laid the foundation of the modern electrochhemical series, and while working as a laboratory assistant discovered the respiratory effects of laughing gas. among his practical inventions was a safety lamp usable in the presence ...
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
The Aufbau Principle: the (n + l) Rule. We've all seen and use the so-called Aufbau Diagram (Figure 1). It is a mnemonic used to remember the order of "filling" of a to mic orbitals during the construction of the ground state electron configurations of the elements. The presentation of th is diagram is largely d is connected from any ...
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
What is the basis for exceptions to the aufbau principle? Filled and half-filled energy sublevels are more stable than partially-filled energy sublevels.
The Aufbau principle holds for almost all elements, especially within the lower atomic numbers. Exceptions are based on the fact that half-full or full shells or subshells are more stable than partially filled ones. When the difference in energy levels between two subshells is small, an electron may transfer to the higher level shell to fill or ...

Minimum Atomic Parameter Basis Sets For Elements 1 54 In A Hartree Fock Setting Reinhardt International Journal Of Quantum Chemistry Wiley Online Library
What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
Aufbau Principle Exceptions . Like most rules, there are exceptions. Half-filled and completely filled d and f subshells add stability to atoms, so the d and f block elements don't always follow the principle. For example, the predicted Aufbau configuration for Cr is 4s 2 3d 4, but the observed configuration is actually 4s 1 3d 5. This actually ...
What is the basis for exceptions to the Aufbau diagram?Get the worksheet from here: https://madecalculators.blogspot.com/2020/11/chemistry-more-questions.html
Ppt Quantum Numbers And Orbital Energies Each Atom S Electron Has A Unique Set Of Quantum Numbers To Define It N L Powerpoint Presentation Id 292706















0 Response to "40 what is the basis for exceptions to the aufbau diagram"
Post a Comment