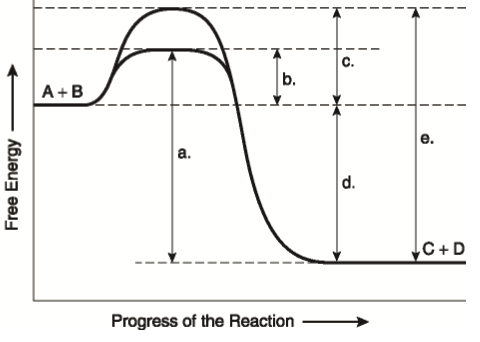
41 the diagram below represents a spontaneous reaction (δg°
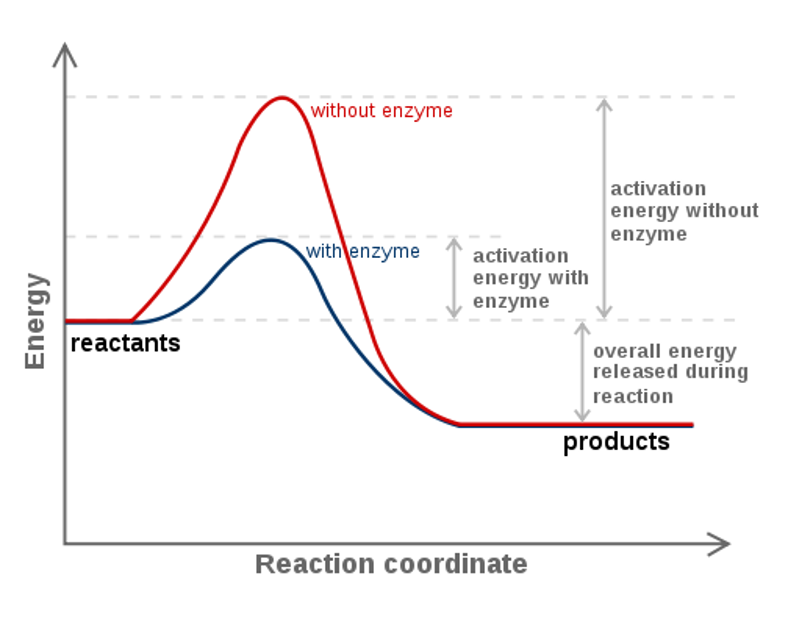
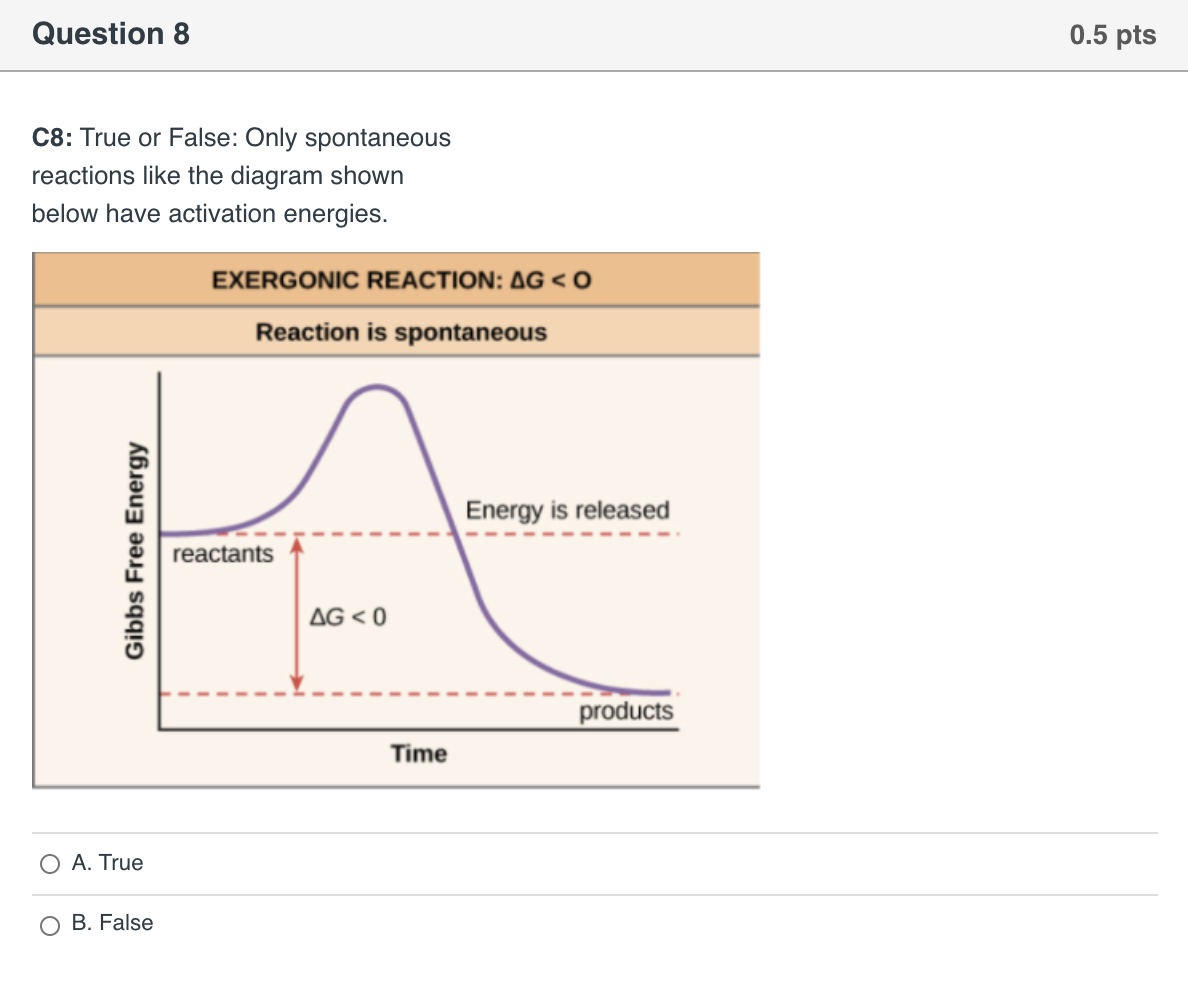
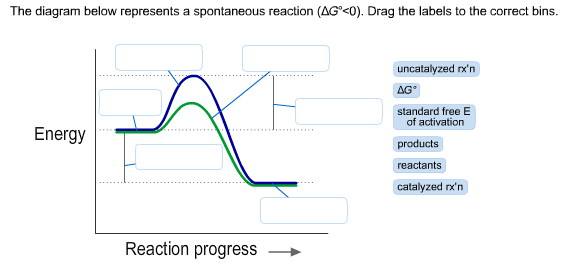
14 Dec 2020 — The diagram below represents a spontaneous reaction. Drag the labels to the correct bins. Is the reaction endothermic or exothermic? The reaction will only be allowed if the total entropy change of the universe is zero or positive. This is reflected in a negative ΔG, and the reaction is called an exergonic process. If two chemical reactions are coupled, then an otherwise endergonic reaction (one with positive ΔG) can be made to happen.
Aug 01, 2017 · (a) Summary of Mg alloy development, and (below) phase diagram for the Mg-Al binary system (extreme left) and Mg-Al-Mn isothermal section (in the middle) as well as a micrograph showing a typical microstructure of an Mg-Al alloy AZ91. Phase diagrams are reproduced based on , . The Mg-Al diagram is reproduced with permission from Elsevier.
The diagram below represents a spontaneous reaction (δg°
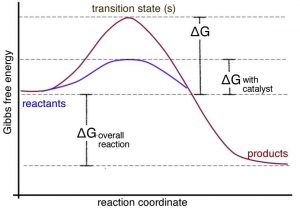
Kenneth W. Raymond · 2009 · ScienceΔG is negative for a spontaneous reaction. ... Using a similar type of drawing, called a reaction energy diagram, we can represent the energy change that ... The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free ...1 answer · 0 votes: Concepts and reason In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous ... 5 Apr 2018 — Problem: The diagram below represents a spontaneous reaction (ΔG°<0). ... So here, we need to fill in the blanks in this diagram.1 answer · Top answer: [readmore]
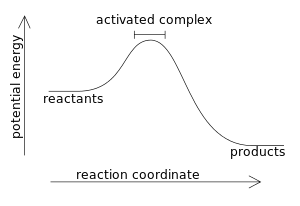
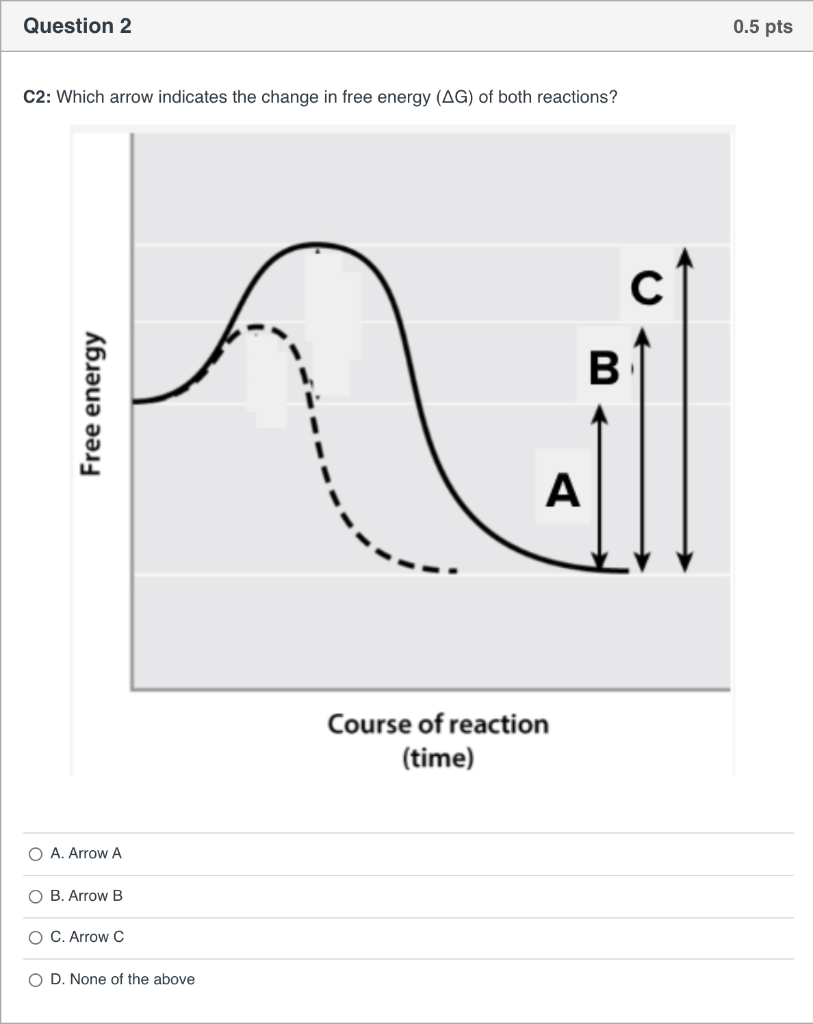
The diagram below represents a spontaneous reaction (δg°. The free energy change, ΔG, of the reaction is negative, so the reaction is considered exergonic and spontaneous. In reaction B, the stability of the substrate is greater than the stability of the product. The free energy change, ΔG, of the reaction is positive, so the reaction is considered endergonic and not spontaneous. A reaction in which the reactants are in standard states, but in which no products have formed, has a ΔGrxn that is more positive than ΔG°rxn. a. A high concentration of reactants relative to products results in a more spontaneous reaction than one in which the reactants and products are in their standard states. (B) The D- and L-Alanine enantiomer pair, upper diagram represents the ball and stick model and the lower diagram represents the line structure. Image (A) from NASA Note that the D- and L-designations are specific terms used for the way a molecule rotates plain polarized light. The Gibbs free energy change (ΔG) and how it's related to reaction ... it takes between starting and final states (the purple lines on the diagrams below), ...
Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n. This problem has been ... Academia.edu is a platform for academics to share research papers. 1) CO 2 + H 2 O ← Carbonic anhydrase H 2 CO 3 {\displaystyle {\ce {CO2{}+H2O<-[{\text{Carbonic anhydrase}}]H2CO3}}} (in lungs ; low CO 2 concentration) (2) The rate of a reaction is dependent on the activation energy needed to form the transition state which then decays into products. Enzymes increase reaction rates by lowering the energy of the transition state. First, binding forms a low ... 5 Apr 2018 — Problem: The diagram below represents a spontaneous reaction (ΔG°<0). ... So here, we need to fill in the blanks in this diagram.1 answer · Top answer: [readmore]
The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free ...1 answer · 0 votes: Concepts and reason In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous ... Kenneth W. Raymond · 2009 · ScienceΔG is negative for a spontaneous reaction. ... Using a similar type of drawing, called a reaction energy diagram, we can represent the energy change that ...

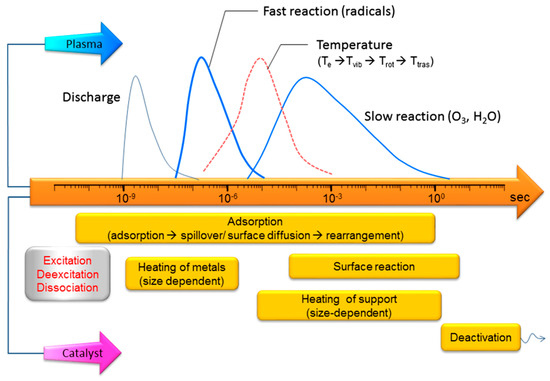
Catalysts Free Full Text Methane To Methanol Through Heterogeneous Catalysis And Plasma Catalysis Html
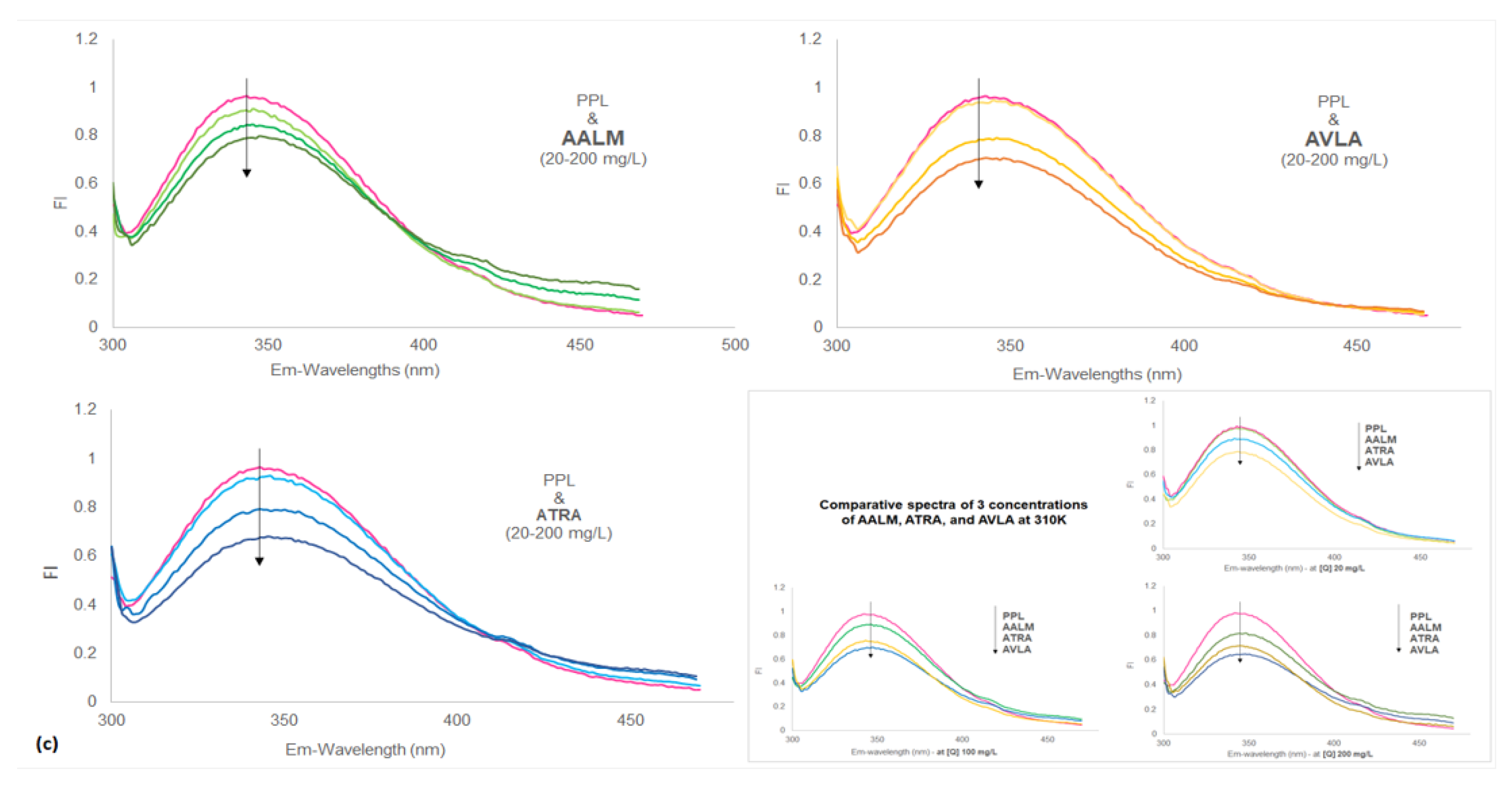
Metabolites Free Full Text Evaluation Of Bioactive Metabolites And Antioxidant Rich Extracts Of Amaranths With Possible Role In Pancreatic Lipase Interaction In Silico And In Vitro Studies Html
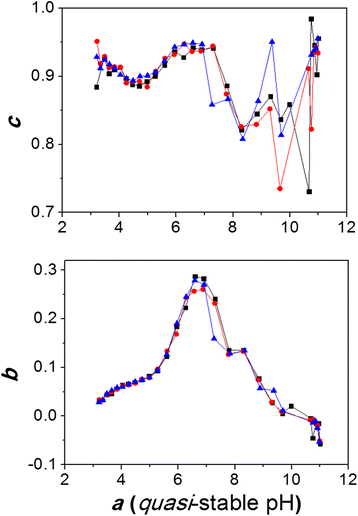
Humic Acid Potentiometric Response Patterns Out Of Equilibrium Properties And Species Distribution Modeling Chemical And Biological Technologies In Agriculture Full Text

In Situ Quantitative Study Of The Phase Transition In Surfactant Adsorption Layers At The Silica Water Interface Using Total Internal Reflection Raman Spectroscopy Physical Chemistry Chemical Physics Rsc Publishing
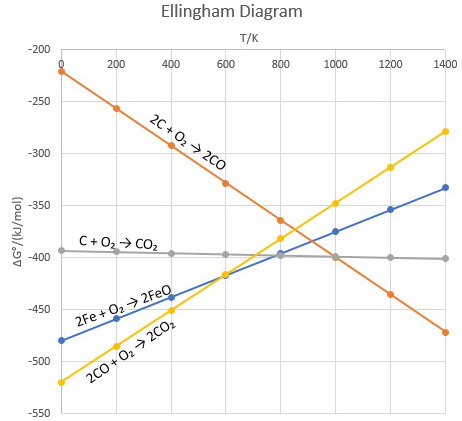
Using Ellingham Diagram How To Determine That In Between C And Co Which Is Better Reducing Agent Socratic

The Reaction Mechanism Of Chiral Hydroxylation Of P Oh And P Nh2 Substituted Compounds By Ethylbenzene Dehydrogenase
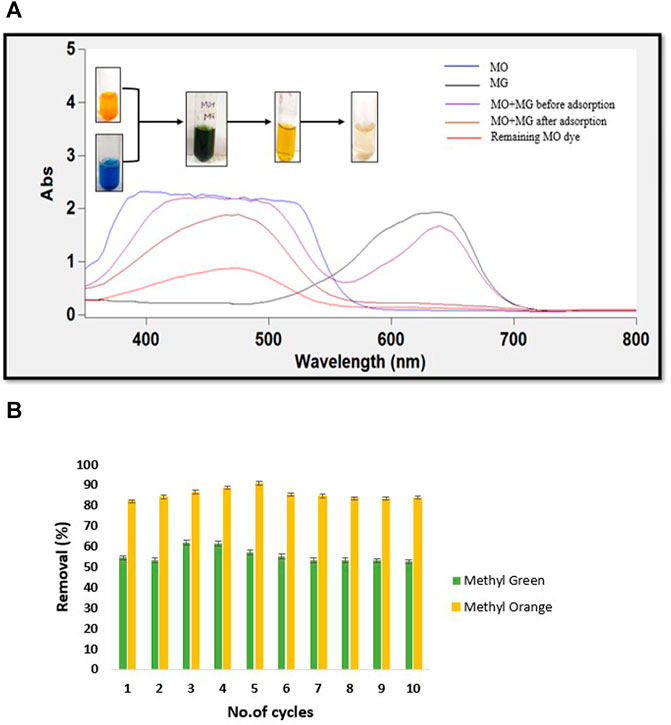
Frontiers Green Synthesis Of Ph Responsive Self Assembled Novel Polysaccharide Composite Hydrogel And Its Application In Selective Capture Of Cationic Anionic Dyes Chemistry
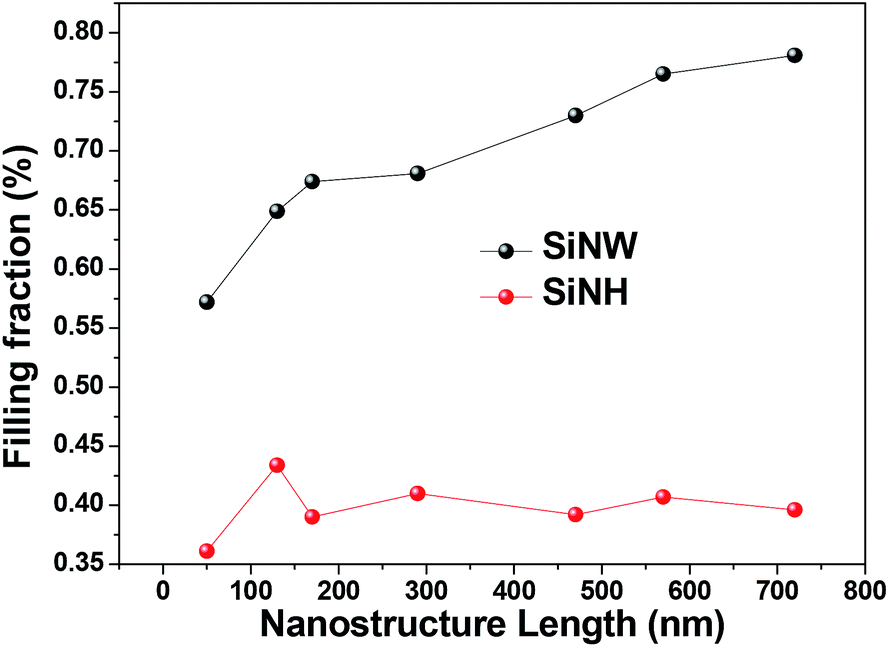
Optical Trapping Enhancement From High Density Silicon Nanohole And Nanowire Arrays For Efficient Hybrid Organic Inorganic Solar Cells Rsc Advances Rsc Publishing Doi 10 1039 C4ra13536a
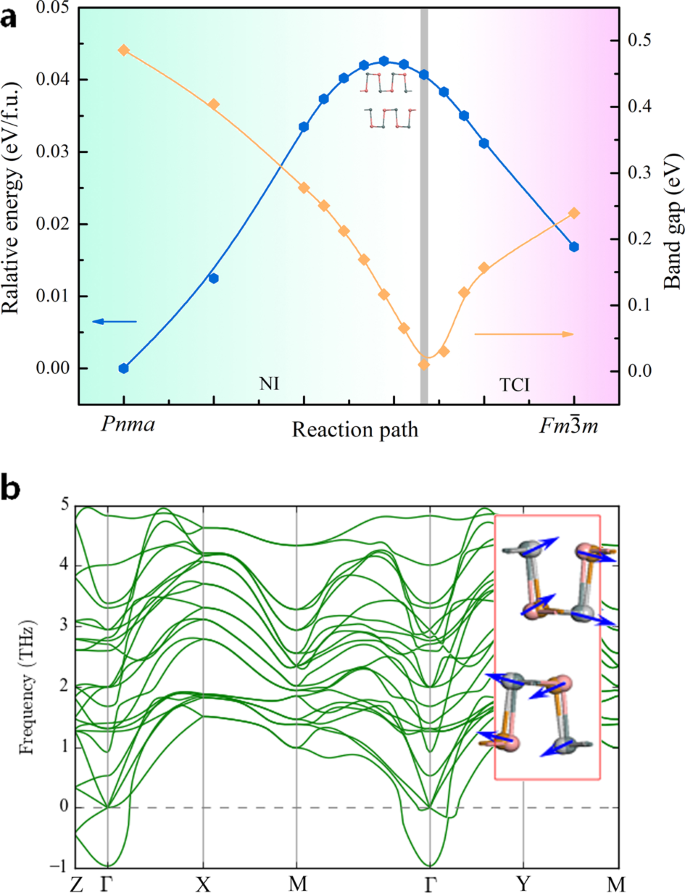
Normal To Topological Insulator Martensitic Phase Transition In Group Iv Monochalcogenides Driven By Light Npg Asia Materials
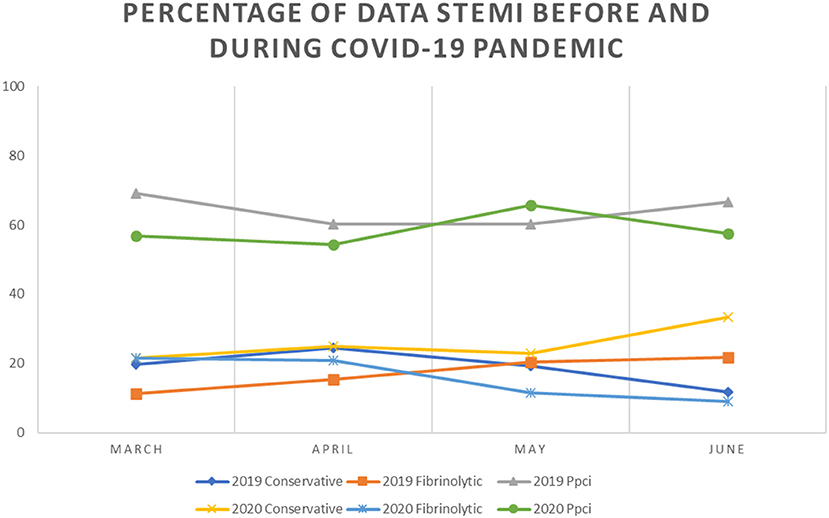
Https Www Frontiersin Org Articles 10 3389 Fpubh 2021 697068 Https Www Frontiersin Org Files Articles 697068 Fpubh 09 697068 Html Image M Fpubh 09 697068 T003 Jpg Table 3 Changes In Social Capital Before And After The Covid 19 Lockdown Among

Phenoxazine Containing Polyaniline Derivatives With Improved Electrochemical Stability And Processability Acs Applied Polymer Materials

Instability Of Amide Bond With Trifluoroacetic Acid 20 Synthesis Conformational Analysis And Mechanistic Insights Into Cleavable Amide Bond Comprising B Troponylhydrazino Acid Acs Omega

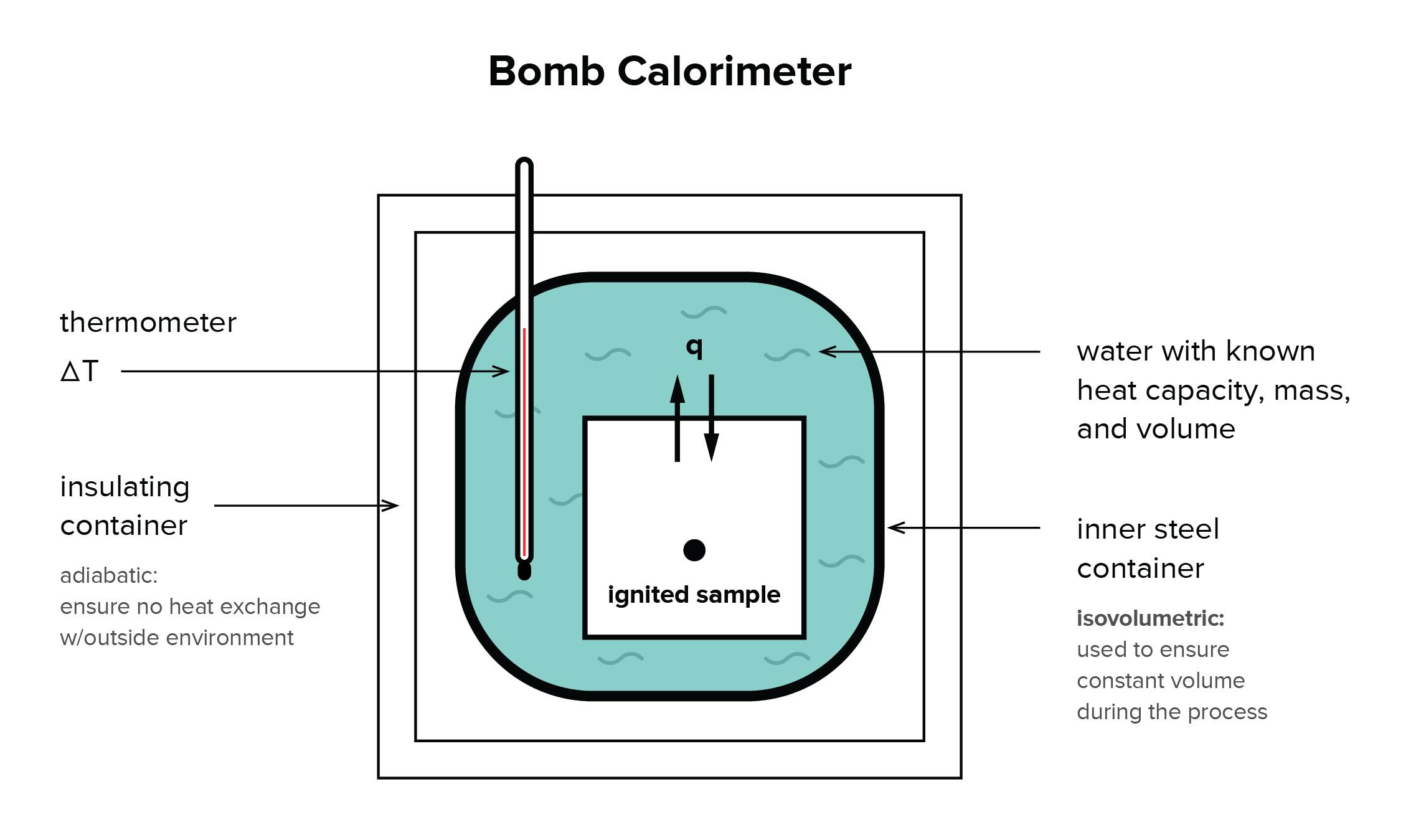










0 Response to "41 the diagram below represents a spontaneous reaction (δg°"
Post a Comment