41 iodine lewis dot diagram
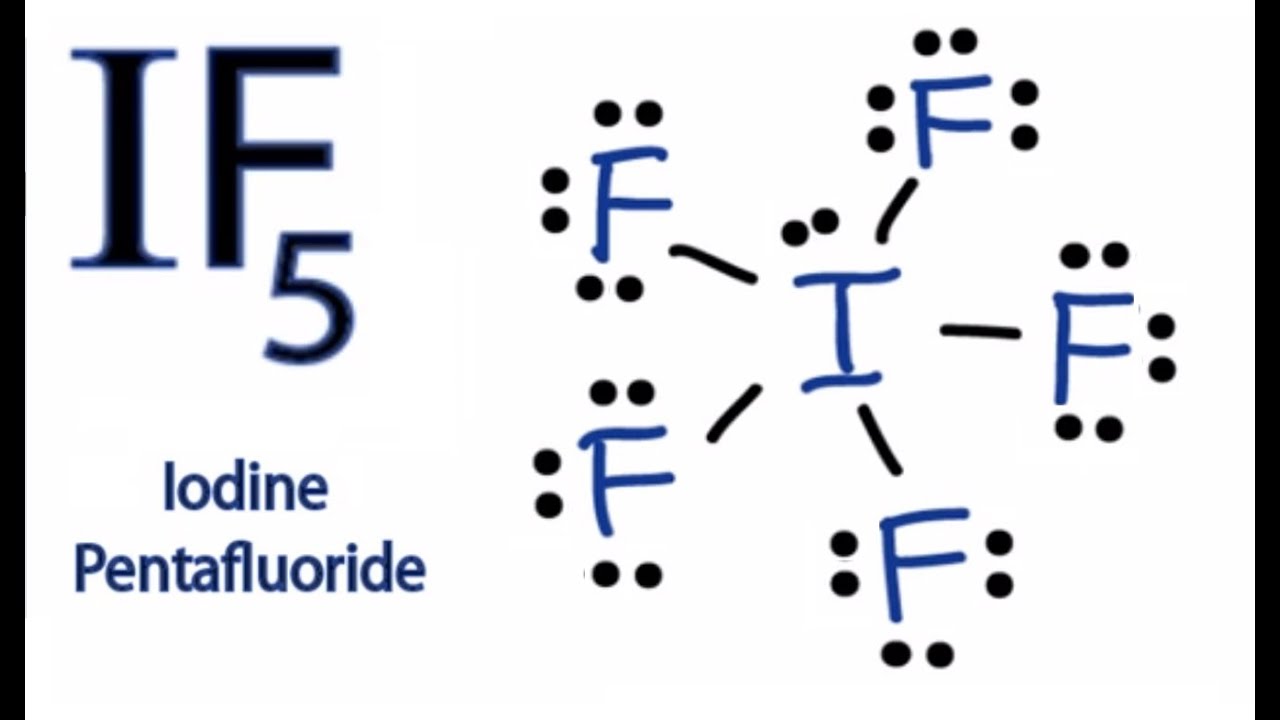
1:46In the Lewis structure of IF7 structure there are a total of 56 valence electrons. IF7 is also called Iodine ...22 Nov 2018 · Uploaded by Wayne Breslyn Lewis Dot Diagrams of Selected Elements. Lewis Symbols: Electron Configuration into Shells: Index Chemical concepts Chemistry of the Elements Periodic Table . HyperPhysics***** Quantum Physics : R Nave: Go Back: Electron Distributions Into Shells for the First Three Periods.
1:23Once we know how many valence electrons there are in I we can distribute them around the central atom with ...9 Aug 2019 · Uploaded by MagnetsAndMotors

Iodine lewis dot diagram
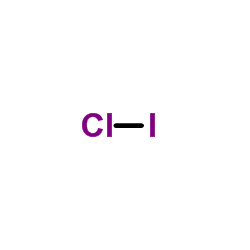
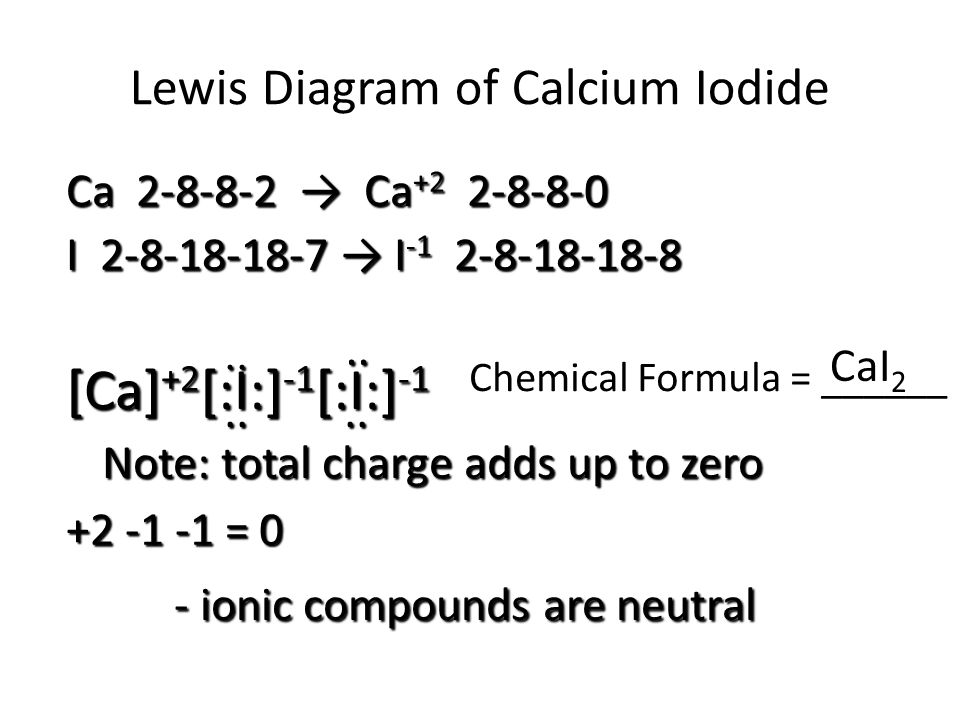
Lewis dot diagram for iodine. Wiki User. ∙ 2009-06-12 05:08:19. See Answer. Best Answer. Copy. Iodine is a diatomic molecule so it's molecules are paired as I2 Iodine has 7 electrons in it's ... Drawing the Lewis Structure for ICl (Iodine Chloride) Viewing Notes: Be careful - the I and the l look similar. The structure is for Iodine (I) Chloride (Cl). For the ICl Lewis structure there are only single bonds. In the Lewis structure for ICl there are a total of 14 valence electrons. Atomic Structure of Iodine ... Electron Configuration: 1s2 2s2p6 3s2p6d10 4s2p6 ...
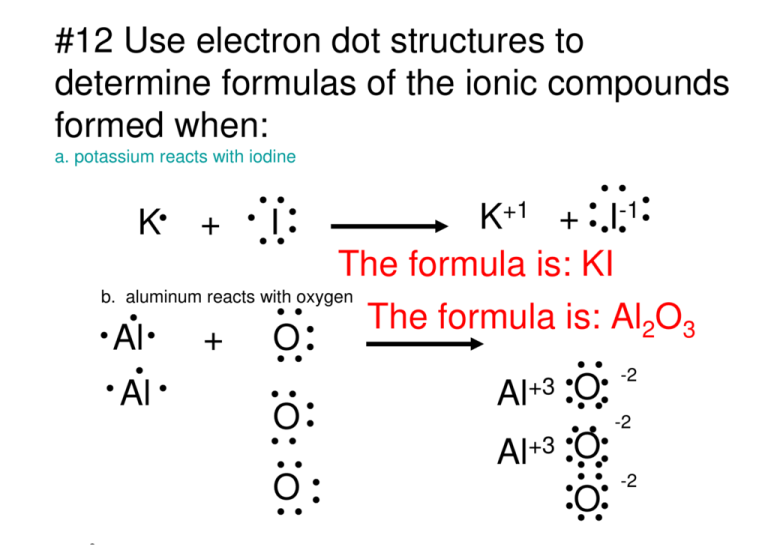
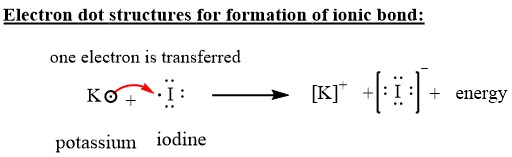
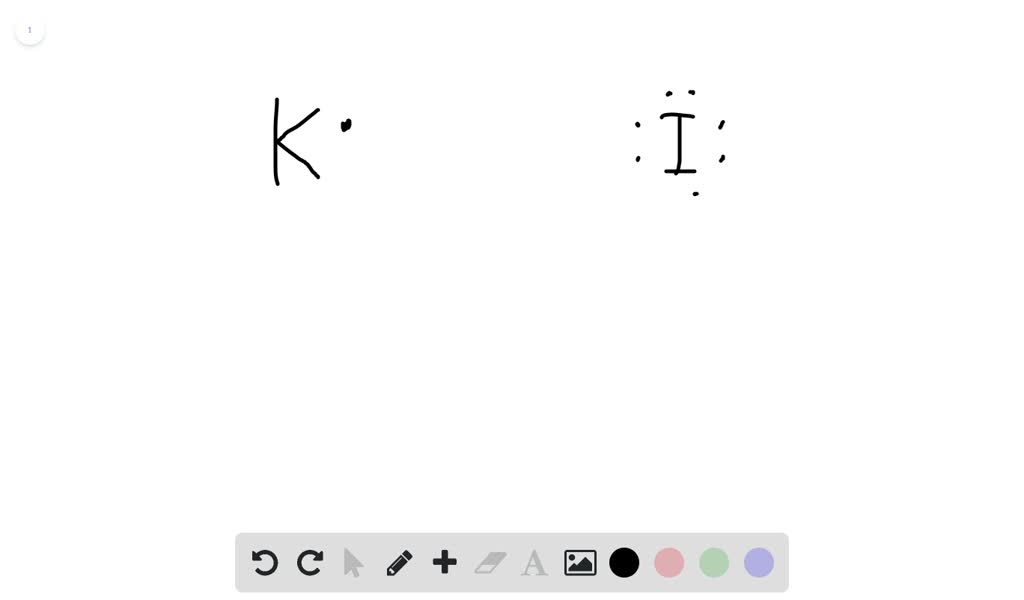
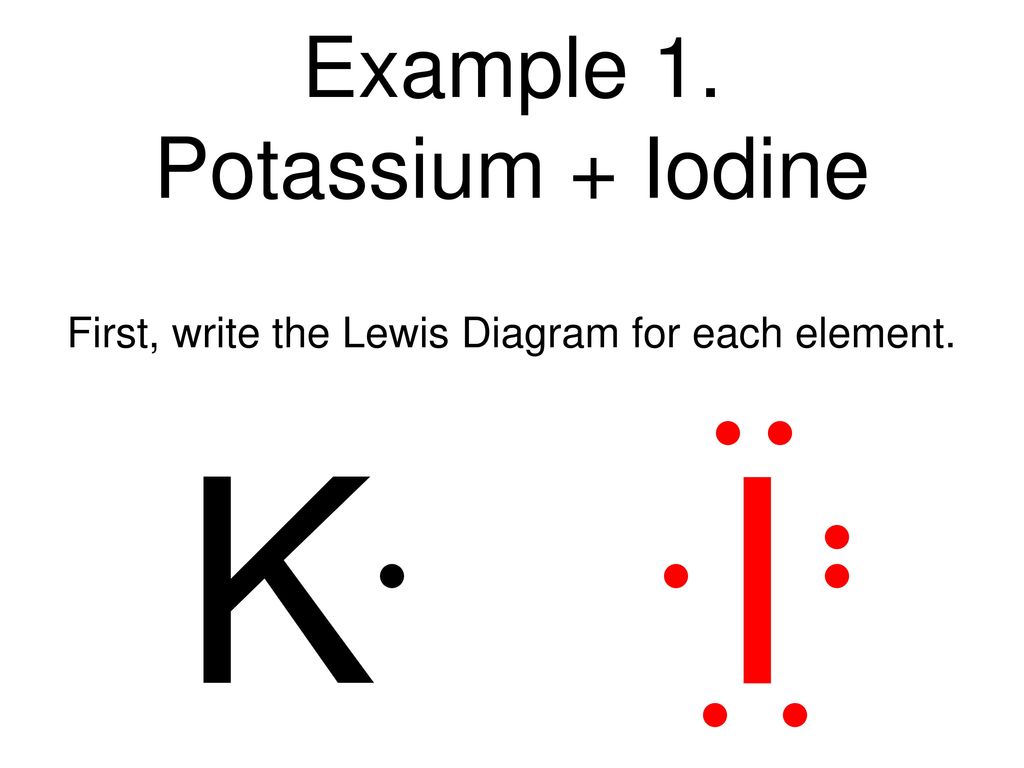
Iodine lewis dot diagram. Sep 21, · Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the element symbol, I, surrounded by seven dots, three on two sides and one on the remaining diagramweb.net: Resolved.Periodic Table of Elements: Iodine - I (diagramweb.net)How do you draw a Lewis dot ... 1:13Once we know how many valence electrons there are in IF we can distribute them around the central atom with ...20 Aug 2019 · Uploaded by MagnetsAndMotors 1:08Because the Iodide ion (I-) has an extra electron (the negative sign denotes an extra electron) we need to ...22 Nov 2018 · Uploaded by Wayne Breslyn 1 answerWhat should the electron dot diagram for iodine look like? Chemistry Drawing Lewis Structures. 1 Answer. Jahan Psyche. Oct 24, 2015. peoi.org.
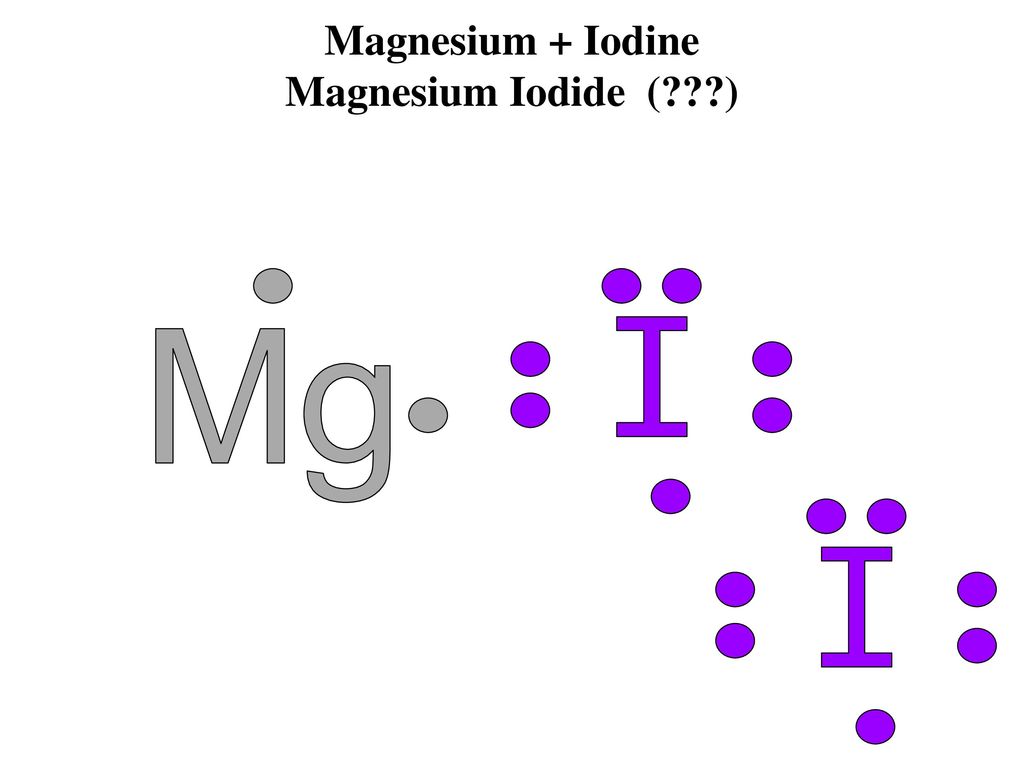
The lewis dot diagram for platinum is a diagram showing bonds electrons of the platinum atom within a molecule. That means it has 7 valence electrons so we have 7. Iodine is in group 7 of the periodic table. Iodine is in group 17 on the periodic table meaning that iodine needs only a single electron to become chemically stable. A step-by-step explanation of how to draw the IF Lewis Dot Structure.For the IF structure use the periodic table to find the total number of valence electron... Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. May 04, · A step-by-step explanation of how to write the Lewis Dot Structure for I2 (Iodine Gas). For the I2 Lewis structure, calculate the total number of valence electrons for the I2 molecule. Transcript: This is Dr. B. Draw a Lewis dot diagram for an iodine molecule. 2. Draw a Lewis dot diagram for a hydrogen molecule. 3. True or false: When drawing a covalent bond, it is proper to use a line connect atoms and a line represents 2 electrons. 4. In your own words, describe the difference between a bond that is nonpolar covalent and polar covalent. Cite one ...
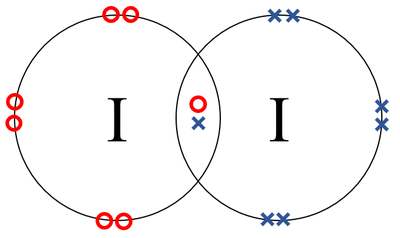
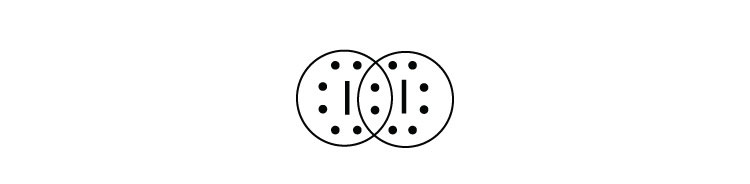
A video explanation of how to draw the Lewis Dot Structure for Iodine Pentabromide, along with information about the compound including Formal Charges, Polar... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... 1:36A step-by-step explanation of how to draw the I2 Lewis Dot Structure (Iodine Gas).For the I2 structure use the ...4 May 2013 · Uploaded by Wayne Breslyn 28 Apr 2021 — Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the element ...
Atomic Structure of Iodine ... Electron Configuration: 1s2 2s2p6 3s2p6d10 4s2p6 ...
Drawing the Lewis Structure for ICl (Iodine Chloride) Viewing Notes: Be careful - the I and the l look similar. The structure is for Iodine (I) Chloride (Cl). For the ICl Lewis structure there are only single bonds. In the Lewis structure for ICl there are a total of 14 valence electrons.
Lewis dot diagram for iodine. Wiki User. ∙ 2009-06-12 05:08:19. See Answer. Best Answer. Copy. Iodine is a diatomic molecule so it's molecules are paired as I2 Iodine has 7 electrons in it's ...

Lewis Dot Structures Lewis Dots Lewis Dot Structures Describe The Covalent Bonding In Molecules They Describe How The Valence Electrons Outermost S Ppt Download

Ion A Charged Atom That Has Gained Or Lost An Electron Atoms That Lose Electrons Become Ions Called Cations Atoms That Gain Electrons Become Ppt Download















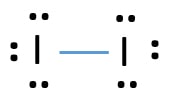
0 Response to "41 iodine lewis dot diagram"
Post a Comment