41 what is a lone pair in a lewis diagram
When you draw the Lewis structure for PF2Cl3, how many single bonds, double bonds, and lone pair electrons reside on the central phosphorus atom? 5 single bonds, 0 double bonds, 0 lone pairs 50. Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
SF4 (Sulfur tetrafluoride) Lewis Structure. SF4 (Sulfur tetrafluoride) molecule contains one sulfur atom and four fluorine atoms. In SF4 lewis structure, each fluorine atom has made single bonds with center sulfur. Also, there is a lone pair on sulfur atom and three lone pairs on each fluorine atom.

What is a lone pair in a lewis diagram
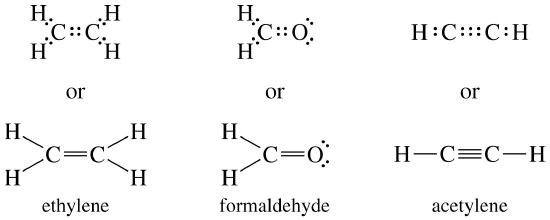
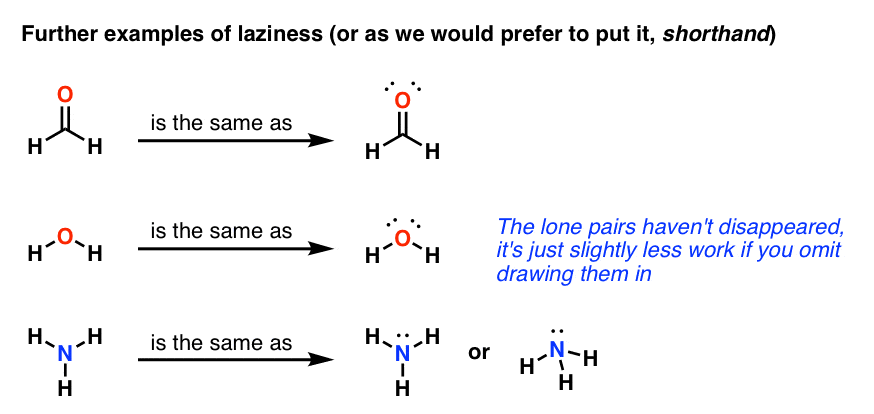
A lone pair consists of 2 electrons in the same orbital from the same atom which are not involved in bonding. Ammonium ion nh4 is a regular tetrahedron. A dash or line is sometimes used to indicate a shared pair of electrons. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding called lone ... SiCl4 lewis's structure is straightforward and very easy to draw. "Lewis diagram describes the chemical bonding of atoms within a molecule". Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. If we can't get a satisfactory Lewis structure by sharing a single pair of electrons, it may be possible to achieve this goal by sharing two or even three pairs of electrons. Example: Consider formaldehyde (H 2 CO) which contains 12 valence electrons. H 2 CO: 2(1) + 4 + 6 = 12 .
What is a lone pair in a lewis diagram. The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond. The basic idea is that in any Lewis structure... ... If there are remaining valence electrons, they must be lone pairs (LPs) around the central atom, ... 13 Feb 2019 — Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of ... The Lewis structure for formaldehyde shows that the oxygen has two lone pairs and a bond to the central carbon. Again, this requires 3 equivalent bonding orbitals , sp 2 hybridization. The electron configuration in the hybridized orbitals shows that to of the orbitals will be occupied by lone pairs of electrons and the third sp 2 orbital will ...
This diagram shows bonds with the help of lines and lone pairs of electrons as dots. The Lewis structure helps with understanding how electrons are distributed within a compound along with its molecular geometry. Besides this, the lewis structure helps with determining the hybridization of the molecule. Lewis structure of O2. The Lewis diagram ... The Lewis dot structures of the individual, non-metal atoms give a good indication of the bonding possibilities for the atoms. Carbon tends to form 4 bonds and have no lone pairs. Nitrogen tends to form three bonds and have on e lone pair. Oxygen tends to form two bonds and have two lone pairs. Lewis structure of BeF2- • valenec electron for all atoms- Be= 2, 2F = 2×7= 14, total electron= 16 electrons • electrons required to comp… View the full answer Transcribed image text : Question 5 of 30 > Draw the Lewis structure for BeF2, including lone pairs. A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps to make a prediction about the geometry of a molecule.
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of ... What are lone pairs and how are they represented in a Lewis dot diagram? What is the Lewis dot diagram for #H_2O#? Is it possible to draw Lewis dot diagrams for ionic compounds? How can I draw a Lewis dot diagram for carbon dioxide? Can Lewis structures predict the shape of a molecule? ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. NF 3 lewis structure. In the lewis structure of NF 3, there are three N-F bonds and one lone pair on nitrogen atom which is the center atom.Each fluorine atom has three lone pairs. Steps of drawing lewis structure of NF 3. You have to follow few steps to draw the lewis structure of NF 3.Because nitrogen trifluoride is a simple molecule, these steps are not complex and do not require all steps ...
Answer (1 of 3): The Lewis structure of ozone (O3) 1. Sum of valence electrons = (6*3) = 18 2. Drawing the bond connectivities: 3. Complete the octets of the atoms bonded to the central atom: 4. Place any leftover electrons (18-16 = 2) on the central atom: 5. Does the central atom have a...
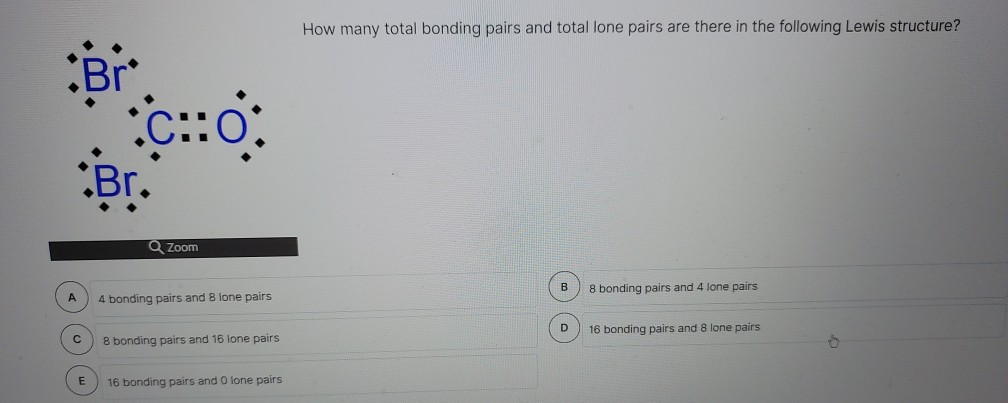
Bonding pairs and lone pairs: since an orbital can hold two electrons we usually talk about electrons in pairs. A bonding pair is the pair of electrons that are being shared. A lone pair are a pair of electrons that are not being shared. Lewis structures are a way of representing the atoms and electrons which constitute a bond.
Question: PF3 Draw the Lewis structure for PF3 in the box at the right, including lone pairs. What is the molecular shape of PF3? O Bent O Tetrahedral O Linear O Trigonal planar The P-F bond in PF3 ispolar Trigonal pyramidal The molecule PF3 is polar What is the F-P-F bond angle? 120 O 109.5。. O 180 $109.5.

Acetone Propanone Ch3coch3 Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure
Lone pair. In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure.
Whereas lone pairs are the pairs of electron on an atom that do not participate in the bonding of two atoms. To identify lone pairs in a molecule, figure out the number of valence electrons of the atom and subtract the number of electrons that have participated in the bonding.
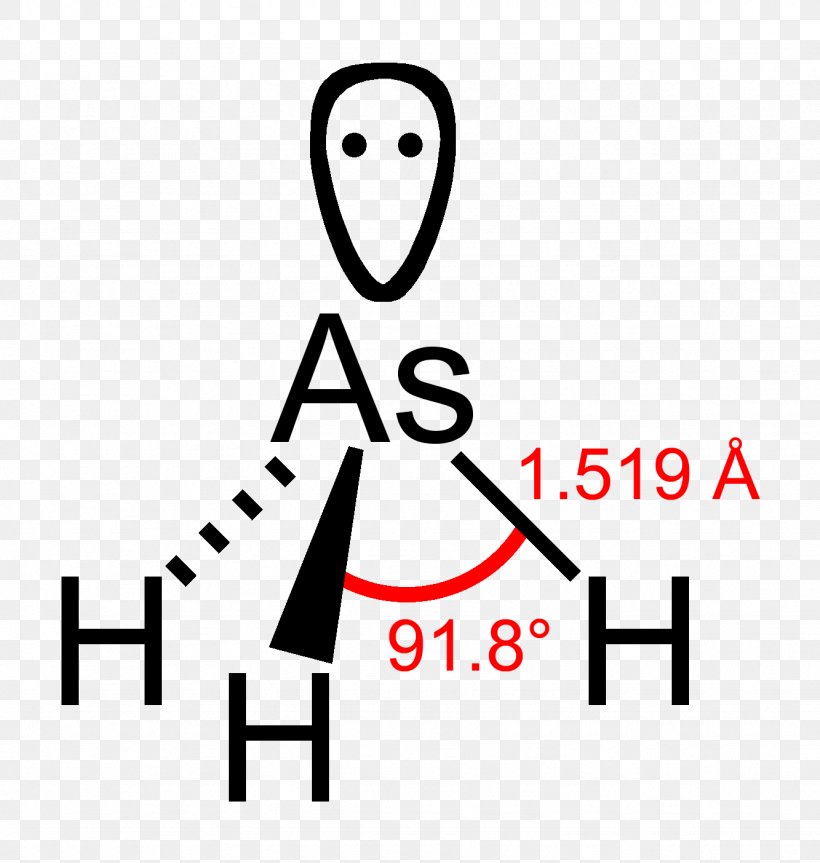
Phosphine Lewis Structure Molecular Geometry Stibine Lone Pair Png 1333x1404px Phosphine Ammonia Area Arsine Atom Download
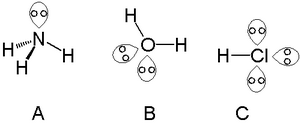
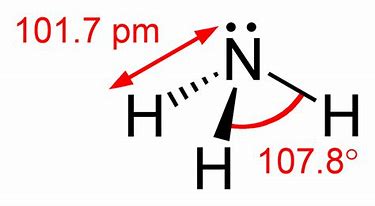
And because the non-bonding nitrogen lone pair lies fairly close to nitrogen it compresses the ∠H − N −H bond down from 109.5∘ to approx. 105∘ in ammonia. On other hand, the lone pair explains the basicity of the ammonia molecule. Ammonium ion, N H + 4, is a REGULAR tetrahedron. And they are normally represented by a double-dot ...
Now, in reality, solid BCl3 is more complicated. The lone pairs on each chlorine atom are attracted to the wide-open slightly-positive charge on the Boron atom, and a Lewis Acid-Base reaction happens: That's fancy chemistry talk for chlorine sharing its lone pair with boron.
Therefore, we have the following structure with a lone pair for this carbanion: To summarize, when asked to determine the number of lone pairs, remember that electrons are associated with a negative charge so if the atom is negatively charged, consider the possibility of having alone pair(s).
A lone pair is an electron pair in the outermost shell of an atom that is not shared or bonded to another atom. It is also called a non-bonding pair. One way to identify a lone pair is to draw a Lewis structure.The number of lone pair electrons added to the number of bonding electrons equals the number of valence electrons of an atom.
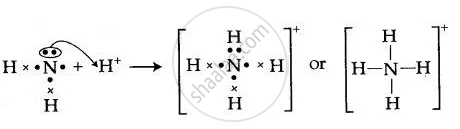
By Drawing An Electron Dot Diagram Show The Lone Pair Effect Leading To The Formation Of Ammonium Ion From Ammonia Gas And Hydrogen Ion Chemistry Shaalaa Com
ClO 3-Lewis structure. Questions. Ask your chemistry questions and find the answers What is the shape around chlorine atom in ClO 3-ion?. There are three σ bonds and a one lone pair around chlorine atom in lewis structure of ClO 3-ion. Therefore, shape of ion is trigonal pyramidal.
Hydrogen Cyanide In this example, HCN, the Lewis diagram shows carbon at the center with no lone electron pairs. The carbon and nitrogen are bonded through a triple bond which counts as "one electron pair". Hence the molecule has two electron pairs and is linear. It boils at 25oC, and thus is a gas a room temperature.
SeF4 lewis structure is made up of one selenium and four fluorine atoms, selenium is the central atom, and fluorine is kept outside in the lewis diagram. There is one lone pair present on the central atom in the SeF4 lewis structure and 12 lone pairs on outer atoms.
XeF2 Lewis Structure. Lewis Structure, also known as electron dot structure, is an essential model of chemical bonding where we use the valence electron concept to schematically sketch a two-dimensional figure of a given molecule. We use dots to represent outer shell electrons and lines to represent the bond type. Xenon is an inert gas element.
If we can't get a satisfactory Lewis structure by sharing a single pair of electrons, it may be possible to achieve this goal by sharing two or even three pairs of electrons. Example: Consider formaldehyde (H 2 CO) which contains 12 valence electrons. H 2 CO: 2(1) + 4 + 6 = 12 .
SiCl4 lewis's structure is straightforward and very easy to draw. "Lewis diagram describes the chemical bonding of atoms within a molecule". Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride.
A lone pair consists of 2 electrons in the same orbital from the same atom which are not involved in bonding. Ammonium ion nh4 is a regular tetrahedron. A dash or line is sometimes used to indicate a shared pair of electrons. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding called lone ...

Ammonium Text Lewis Structure Ammonia Ion Polyatomic Ion Ammonium Ironiii Sulfate Ammonia Solution Lone Pair Ammonium Lewis Structure Ammonia Png Pngwing

Phosphoric Acid Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic

How Many Covalent Bonds And How Many Lone Pairs Are There In The Lewis Structure Of Co 3 2 Study Com

Ammonium Sulfate Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic

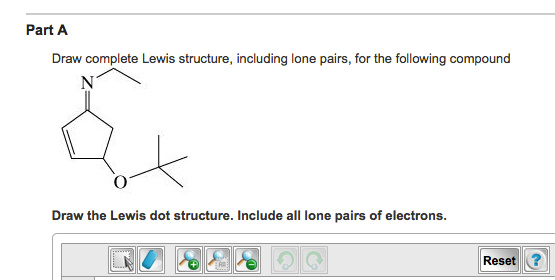
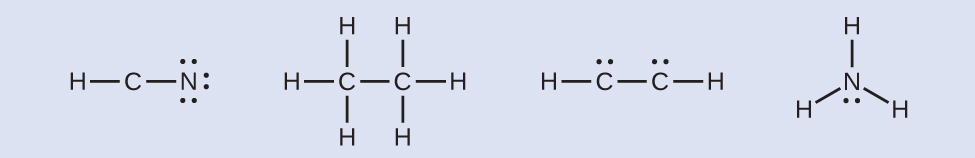



/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)



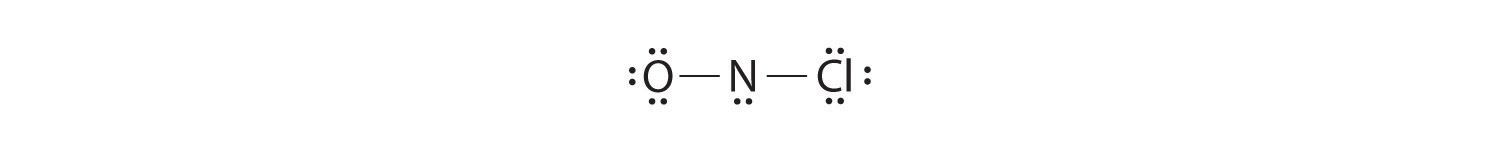








0 Response to "41 what is a lone pair in a lewis diagram"
Post a Comment