42 molecular orbital diagram for n2 2-
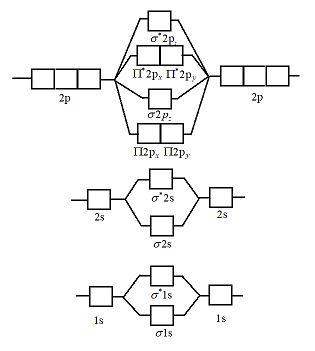
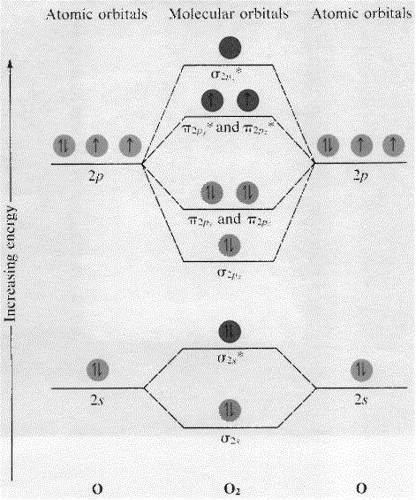
Here is the full molecular orbital diagram for N 2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively. The 2 and 3 orbitals correspond to the non-bonding ... the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
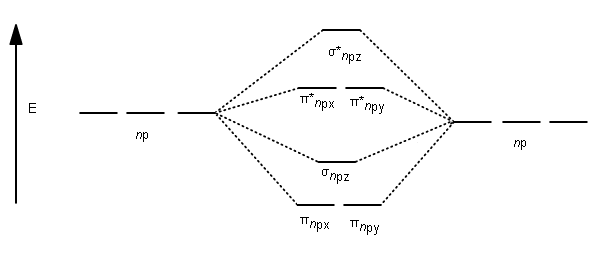
Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules. The same method can be applied to for other diatomic molecules, but involving more than the 1 s atomic orbitals.

Molecular orbital diagram for n2 2-
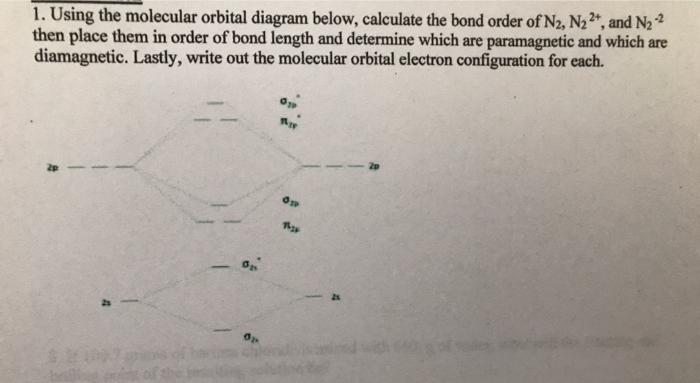
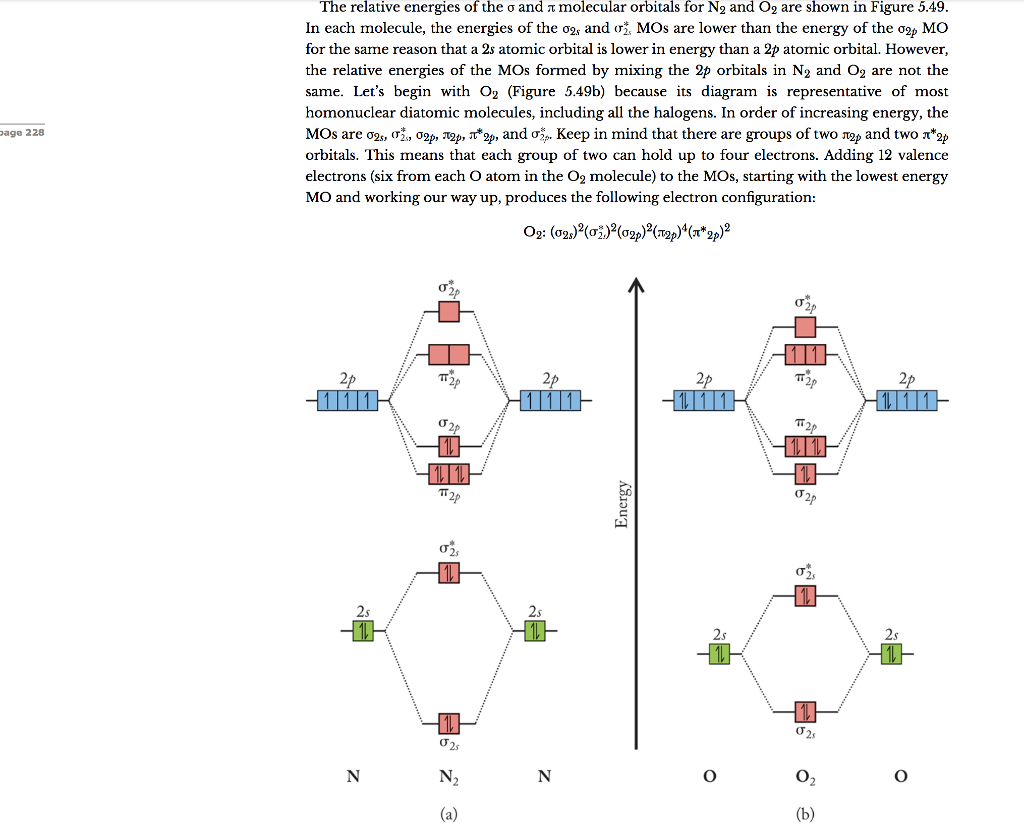
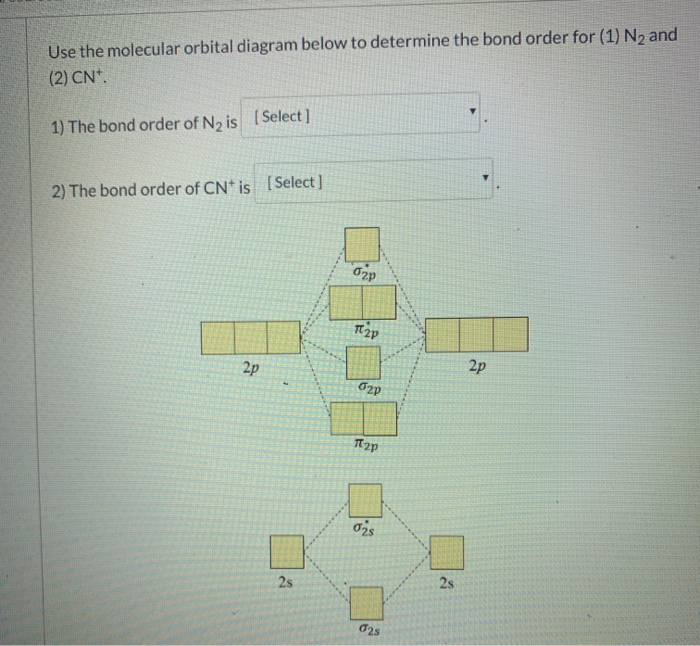
From the molecular orbital diagram of N 2, predict its bond order and whether it is diamagnetic or paramagnetic. Answer: N 2 has a bond order of 3 and is diamagnetic. Example 3. Ion Predictions with MO Diagrams Give the molecular orbital configuration for the valence electrons in C 2 2 ... Answer (1 of 2): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ... Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ...
Molecular orbital diagram for n2 2-. N2 2 molecular orbital diagram. Bond order is 3 and it is paramagnetic. Molecular orbital diagram n2 2. Answers to molecular orbitals problem set 1. In n 2 and in most other diatomic molecules no ns co cs there are 4 sigma symmetry molecular orbitals made from a mixing of the 2s and 2p z atomic orbitals on each atom. Formula used: bond order$ = \dfrac{{{\text{bonding electrons - antibonding electrons}}}}{2}$ Complete step by step answer: Molecular orbital diagram explains the chemical bonding in the molecules in terms of molecular orbital theory. In a molecular orbital diagram, the diagram of molecular orbital energy levels is shown as horizontal lines. N2 2- Molecular orbital Diagram. molecular orbital mo diagram of n2 molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you sigma2s 2 sigma2s 2 pi2p 4 mo diagram for n2 molecular orbital there are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc e is for the elements up to nitrogen the other is for after. 14+ N2 Mo Diagram. With mo diagrams, we can predict the number of bonds in diatomic molecules. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2). N2 2 Molecular Orbital Diagram — UNTPIKAPPS from www.untpikapps.com Thus if we know…
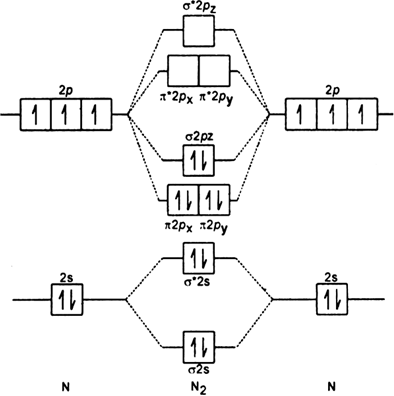
Nov 02, 2015 · If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion. It's not ... Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ... N2 molecular orbital diagram. The two dots represent the n nuclei. Molecular orbital diagram of n2 ionn2 2 molecular orbital diagramn2 molecular orbital diagram bond ordern2 molecular orbital energy diagramn2. The nitrogen has low barrier between the s and p orbitals so they mix a bit this creates the pattern 2pi sigmap. Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)).Fill from the bottom up, with 9 valence electrons total.Bonding Order is 2.5, so it is unstable, ...
Molecular orbital diagram for n2. Then just fill the. One is for the elements up to nitrogen. For the second period elements the 2s and 2p orbitals are important for mo considerations. Since the s h h orbital shows a decrease in bonding between the two nuclei. Perpendicular to these in the yz plane the 2py orbitals on each atom combine to make ... The molecular orbital energy level diagram of N2 is given in Fig.. Is N2 2+ paramagnetic or diamagnetic? Hear this out loudPauseThus, N+2 has a paramagnetic configuration due to the unpaired σ2pz electron. Is N2+ stronger than N2? Hear this out loudPauseBoth N2+ and N2 - have 5 net bonding electrons and so both have weaker bonds than N2. Why ... Molecular orbital (MO) diagram for N2 and N2^-Ask Question Asked 6 years, 3 months ago. Active 3 years, 10 months ago. Viewed 118k times 24 7 $\begingroup$ I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. ... Now note that even in this advanced ... Draw the molecular orbital diagram for n2 ion and calculate the bond order. Introduction To Molecular Orbital Theory This is the reasoning for the rearrangement from a more familiar diagram. N2 molecular orbital diagram. First though notice that the p orbitals are supposed to be degenerate. Consider the two atoms along the z axis.
Molecular orbital diagram for carbon dimer c2. Molecular orbital diagram for n2 o2 c2 f2 also h2o. Fill from the bottom up with 8 electrons total. Mo diagram s can be used to deduce magnetic properties of a molecule and how they change with ionization. O2 2 Molecular Orbital Diagram Fabulous Electron Molecular Orbital. Molecular orbital diagram for c2.This video shows the mo diagrams of the c2 ...
Molecular Orbital Diagram of N2 Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.

Use The Molecular Orbital Energy Level Diagram To Show That Cbse Class 11 Chemistry Learn Cbse Forum
Feb 03, 2019 · The diagram above is the molecular.N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the energy than the atomic and form.
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level ...
On a very general basis, electrons are not assigned to individual bonds between atoms, but they move under the influence of the nuclei in the whole molecule. Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ .
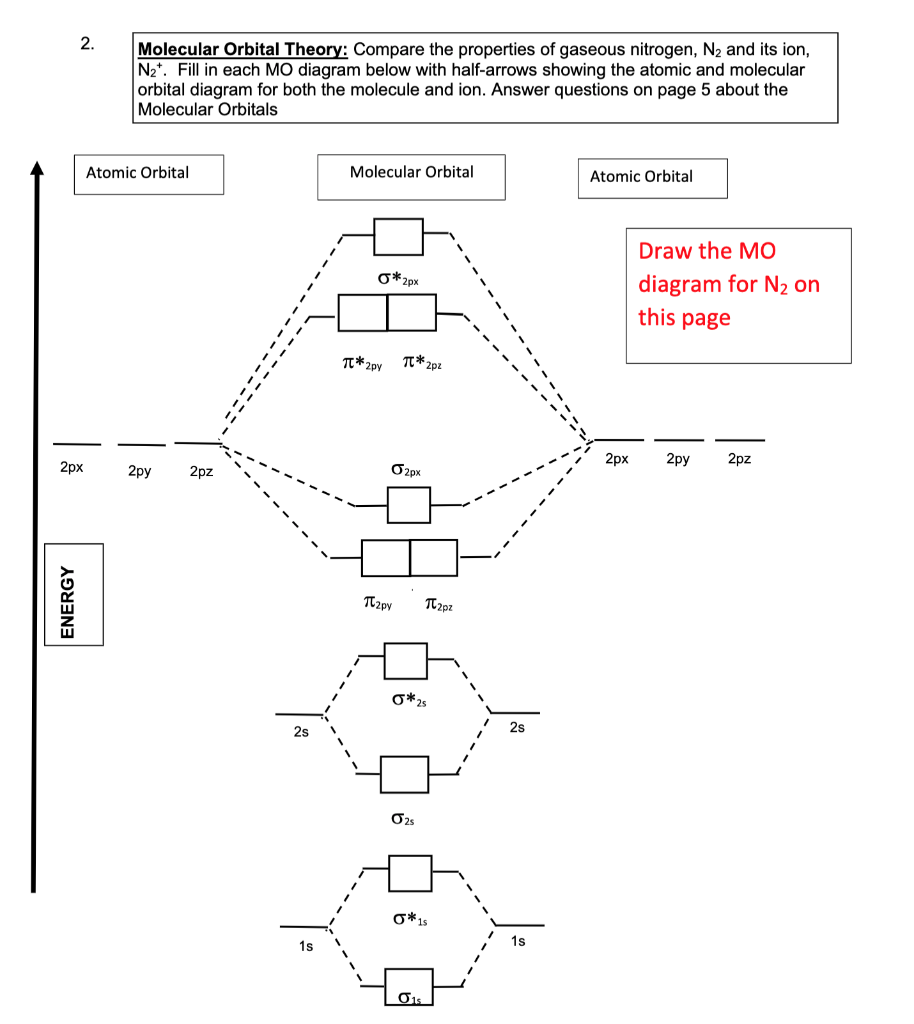
Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric.
Answer (1 of 2): Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma...
When two carbons atoms bond, the pi(2p) bonding molecular orbital s are lower in energy than the sigma(2p) bonding orbital s. C2 (2-) has a bond order of 3, so i... Molecular orbital diagram for n2 o2 c2 f2 also h2o. Molecular orbital diagram for carbon dimer c2.Mo diagram s can be used to deduce magnetic properties of a molecule and how they change with ionization.
Write the molecular orbital diagram of N2+ and calculate their bond order. Asked by sonkarshiva009 | 13th Mar, 2019, 05:47: PM. Expert Answer: Electronic configuration of N-atom(Z=7) is ...
Draw The Molecular Orbital Diagram Of N2 Also Find Its Bond Order And Magnetic Character Chemistry Topperlearning Com 4s4p942zz
Draw the molecular orbital diagram for N2. Label all of the atomic orbitals and molecular orbitals and put the correct number of electrons in. You do not need to draw the shapes of any of the orbitals. a) MO diagram b) Based on your MO diagram, is N2 diamagnetic or paramagnetic? c) Calculate the bond order for N2.
Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ...
Answer (1 of 2): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ...
From the molecular orbital diagram of N 2, predict its bond order and whether it is diamagnetic or paramagnetic. Answer: N 2 has a bond order of 3 and is diamagnetic. Example 3. Ion Predictions with MO Diagrams Give the molecular orbital configuration for the valence electrons in C 2 2 ...

Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic N22 B22 Homeworklib
























0 Response to "42 molecular orbital diagram for n2 2-"
Post a Comment