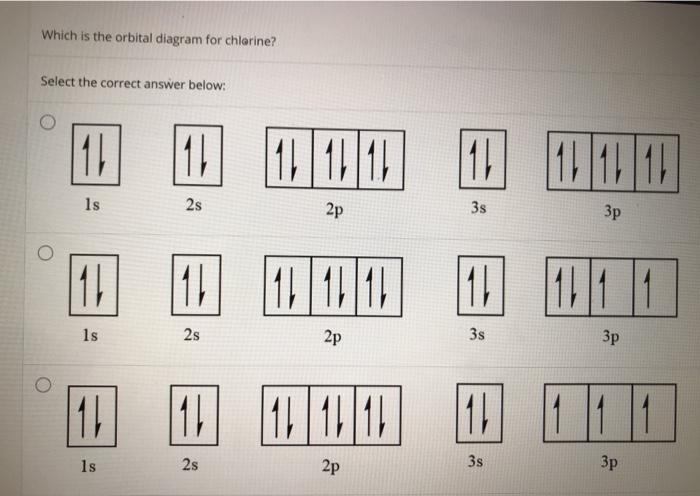
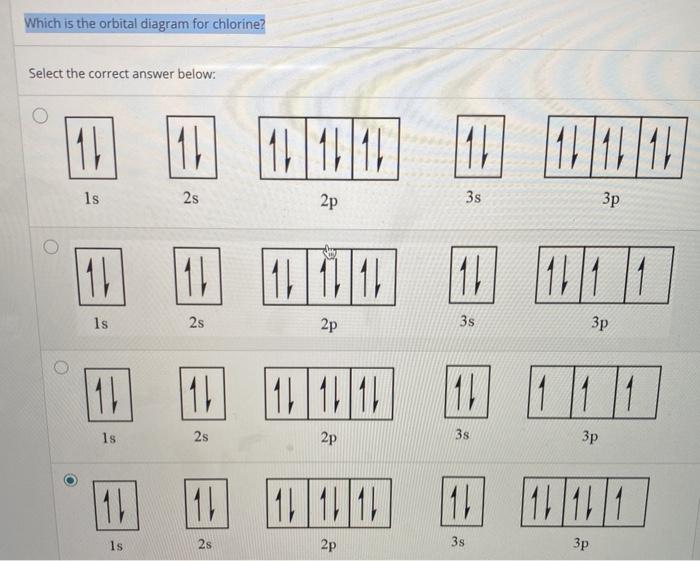
37 orbital diagram for chlorine
FREE Answer to Create the atomic orbital diagram for chlorine. In a chlorine atom, which subshells contain valence electrons?1 answer · 0 votes: Concepts and reason An orbital could be defined as the distribution of an electron’s probability density in an atom, centered at the nucleus. The ...
Draw the atomic orbital diagram for chlorine. Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems Q. Construct the orbital diagram of each atom or ion.TiTi2+Ti4+ Q. Write the corresponding electron configuration for the following pictorial ...
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...

Orbital diagram for chlorine
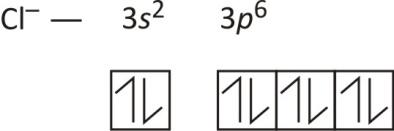
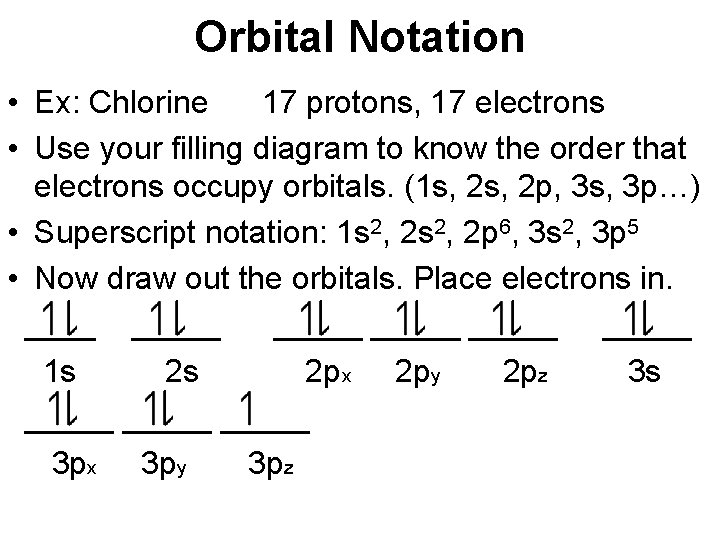
Following these rules, we can write the electron orbital notation of Cl−1 as: To draw this as a diagram, draw a circle representing an orbital for every two electrons, and fill them up one by one with lines representing electrons. Note that you cannot add a second line to an orbital until all the other orbitals in the same letter block (s,p,d ...
Give the orbital diagram for an atom of {eq}Cl {/eq}. Electron Configuration: We use three rules to help determine how to draw the orbital diagrams (and thus get the electron configuration) for atoms:
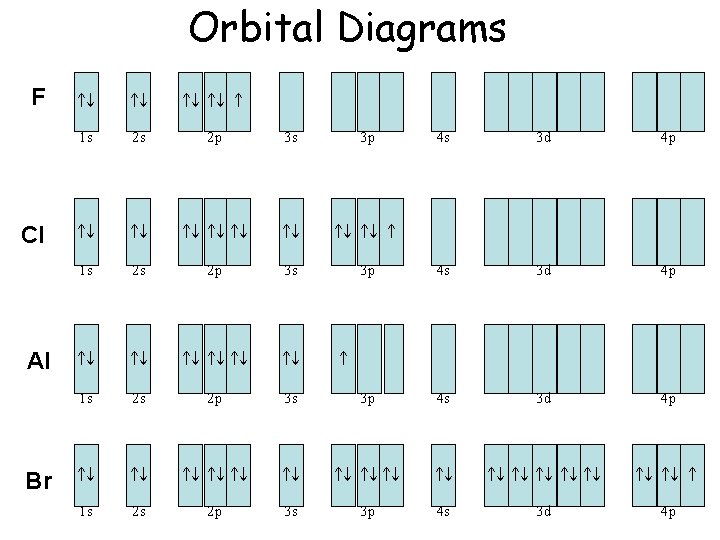
Chlorine (Cl) has an atomic mass of 17. Find out about its chemical and ... Electron Configuration, [Ne] 3s2 3p5. 1s2 2s2 2p6 3s2 3p5. Orbital Diagram.
Orbital diagram for chlorine.
A step-by-step description of how to write the electron configuration for Chlorine (Cl). In order to write the Cl electron configuration we first need to kn...
This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3 p orbital. When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals.
Answer: There is a MO energy diagram here: File:HCl mo diagram.png Mixing hydrogen 1s orbital with chlorine 3s and 3px, 3py and 3pz orbitals, one can obtain following MOs (listed from the least to the most energetic orbitals): 1. one non-bonding sigma MO, mainly chlorine 3s orbital, with two el...
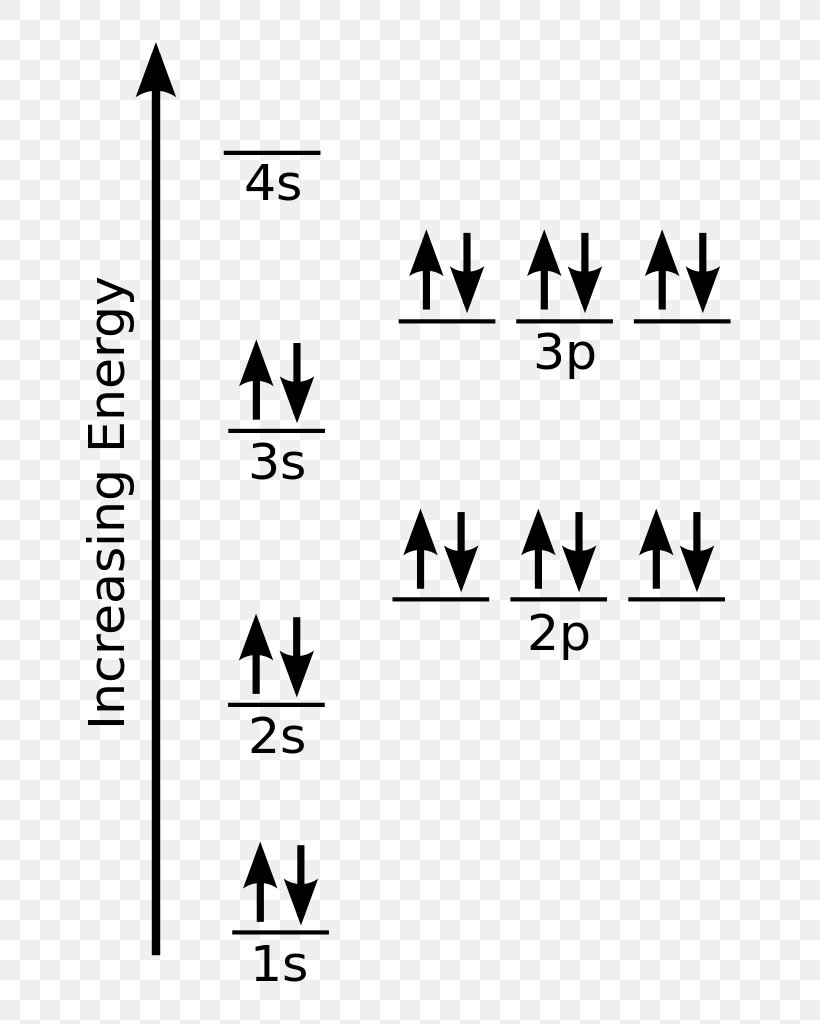
An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Each orbital can hold 2 electrons and each arrow ...
In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Orbital Diagram for Magnesium (Mg) Electron configuration of magnesium ion(Mg 2+). Ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2.After the electron configuration, the last shell of the magnesium atom has two electrons.
Figure 4.10.6: Molecular Orbital Energy-Level Diagram for HCl. The hydrogen 1 s atomic orbital interacts most strongly with the 3 pz orbital on chlorine, producing a bonding/antibonding pair of molecular orbitals. The other electrons on Cl are best viewed as nonbonding.
orbital and energy match, therefore very low HOMO and high LUMO energy! σ C-Cl! σ* C-Cl! C (sp3)! In a C-Cl bond, an sp3 orbital from carbon is still being mixed so same energy level! It is mixed, however, with a p orbital on chlorine which is much lower in energy (more electronegative)! Cl (p)! Poor energy match means orbitals do not
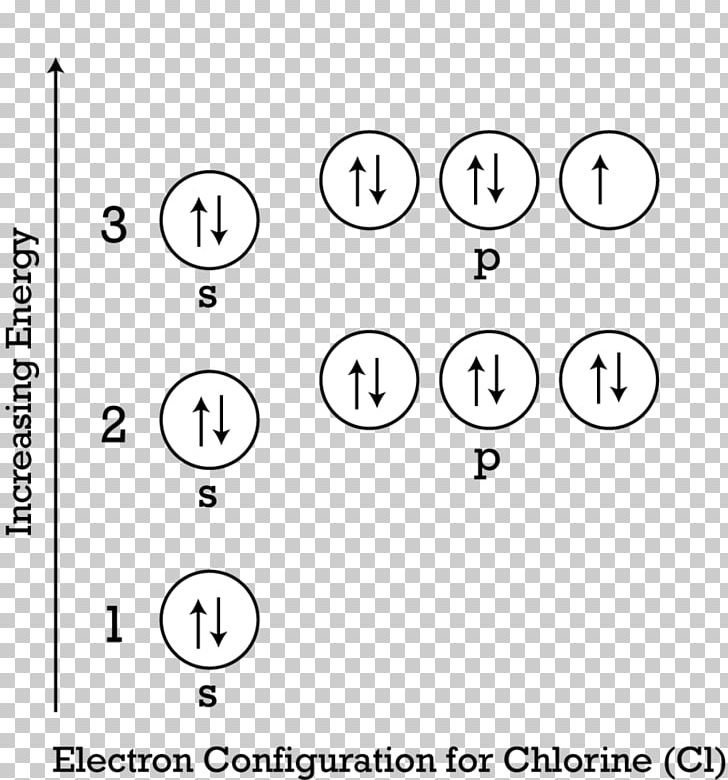
Valence electrons. Orbital diagram. ... Orbital diagram of the Chlorine atom. Distribution of electrons over energy levels in the Cl atom 1-st level (K): 2
Abbreviated orbital diagram of chlorine Description Greenish-yellow, disagreeable gas. Never found in free form in nature. Uses Used in water purification, bleaches, acids and many, many other compounds such as chlorofluorocarbons (CFC). Sources Salt (sodium chloride, NaCl) is its most common compound.
Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 4.
Using the electronic configuration of Cl, we can construct the atomic orbital diagram of Cl, where orbitals are arranged from bottom to top in increasing order of their energies, from 1s to 3p,...
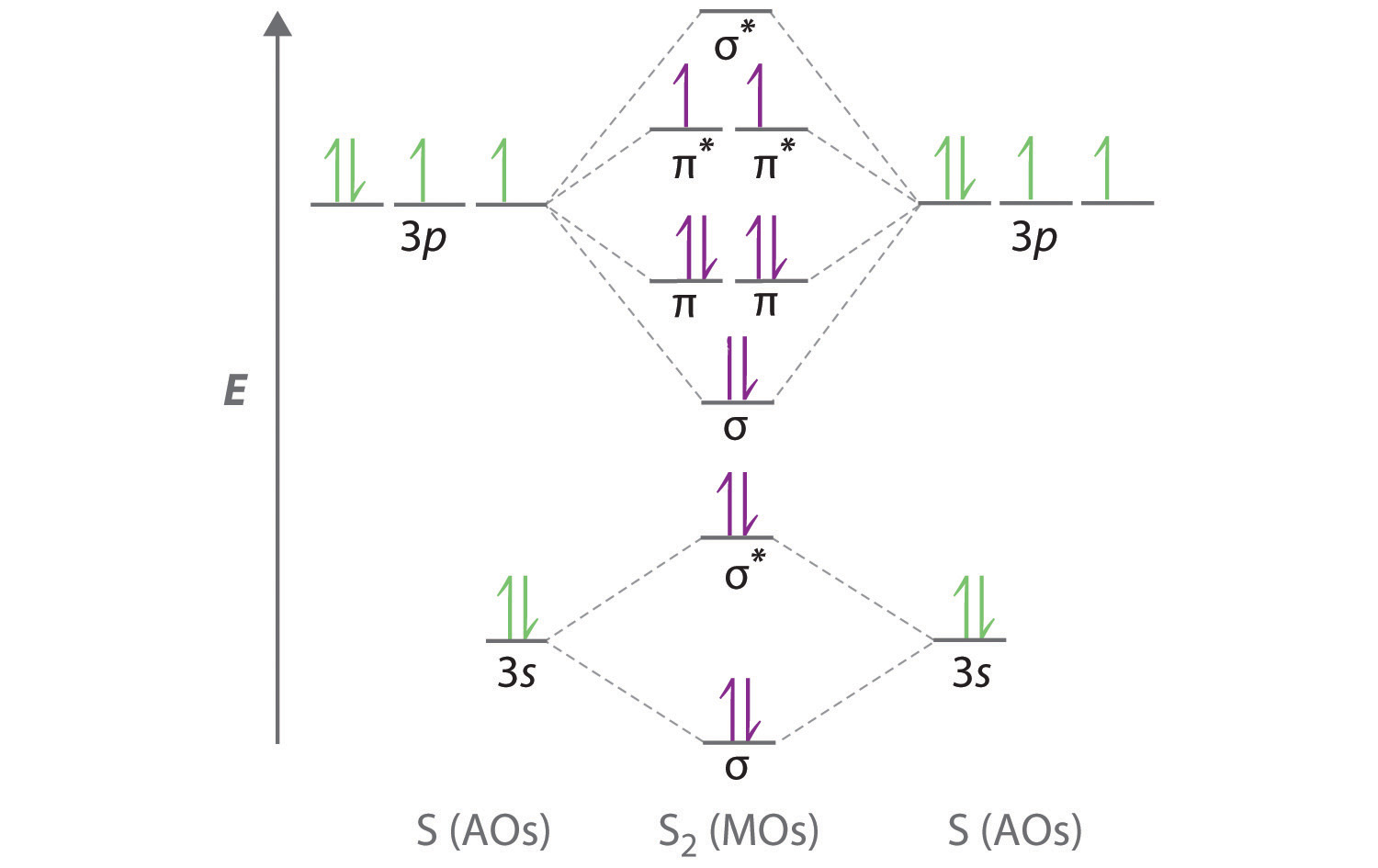
Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F.
Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram ...
Jan 26, 2021 — For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are 17 ...
Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl have comparable energy with the H electron and therefore are allowed to mix.
What is the molecular orbital diagram for HCL? Therefore, the HCL molecule has 8 pairs (1s, 2s, 2px,2py,2pz,3s,3px and 3py) of non-bonding (nb) electrons and one bonding (sigma) orbital having two electrons. The sigma antibonding orbital will be empty. The nb electrons would reside on Cl atom. How to draw the MO diagram for HCL?
Orbital diagram of chlorine 17 1 Know the formula. In molecular orbital theory, the binding order is defined as half the difference between the number of bonding and antibonding electrons. Binding order = [(Number of electrons in binding molecules) - (Number of electrons in antibonding molecules)]/2. 2 Know that the larger the binding order ...
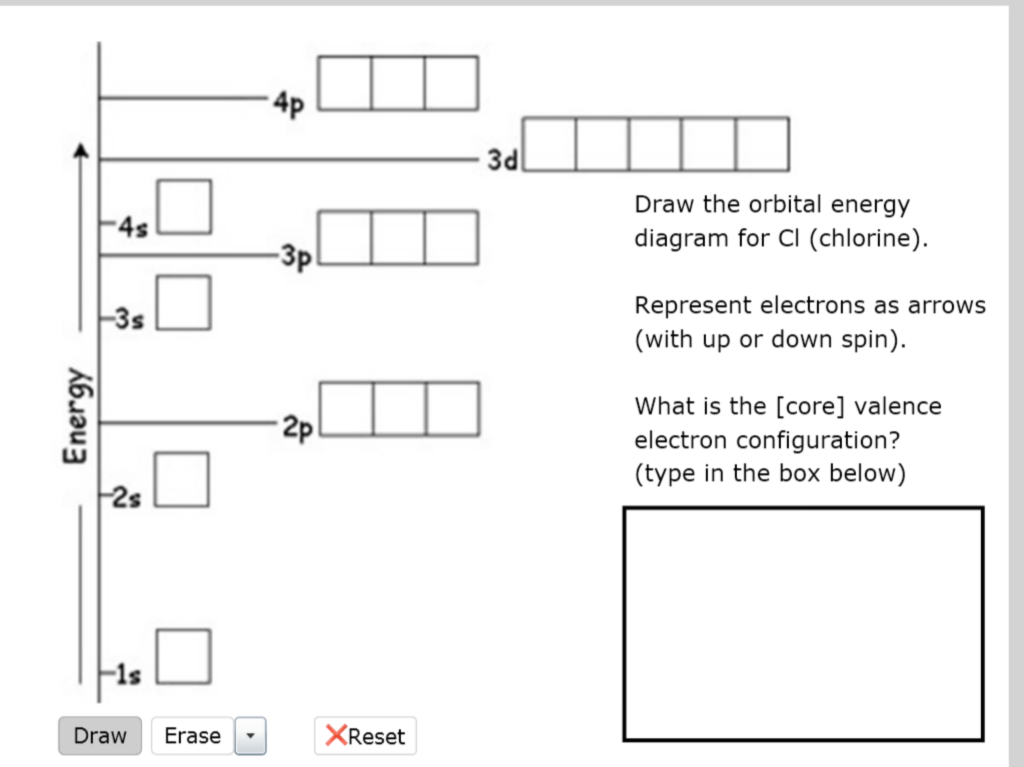
Solved 4p 3d Draw the orbital energy diagram for Cl | Chegg.com. Science. Chemistry. Chemistry questions and answers. 4p 3d Draw the orbital energy diagram for Cl (chlorine) -4s 3p Represent electrons as arrows (with up or down spin) What is the [core] valence electron configuration? (type in the box below) 2pL LU 2s -1s ×Reset Draw Erase.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
The electron configuration of sodium is 1s 2 2s 2 2p 6 3s 1. There is an electron in the last orbit of sodium (3s 1 ). The sodium atom leaves an electron in its last orbit and turns into a cation. Na - e - → Na +. Therefore, the electron configuration of sodium ion (Na +) is 1s 2 2s 2 2p 6.
Cl2 molecular orbital diagram. In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. Energy of. Cl atom has 17 electrons, so chlorine molecule has (Cl2) has 34 electrons. so, bond order of chlorine molecule is 1. How do I determine the bond order using the Lewis structure?.
Create the atomic orbital diagram for chlorine. In writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. The next six electrons will go in the 2p orbital. One electron moves from the sodium atom and attaches to the chlorine atom to fill its outer orbital.
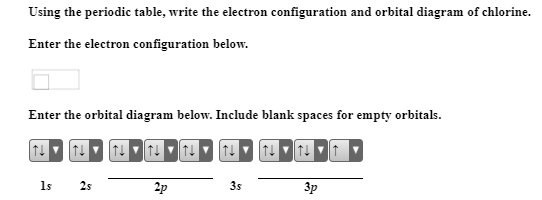
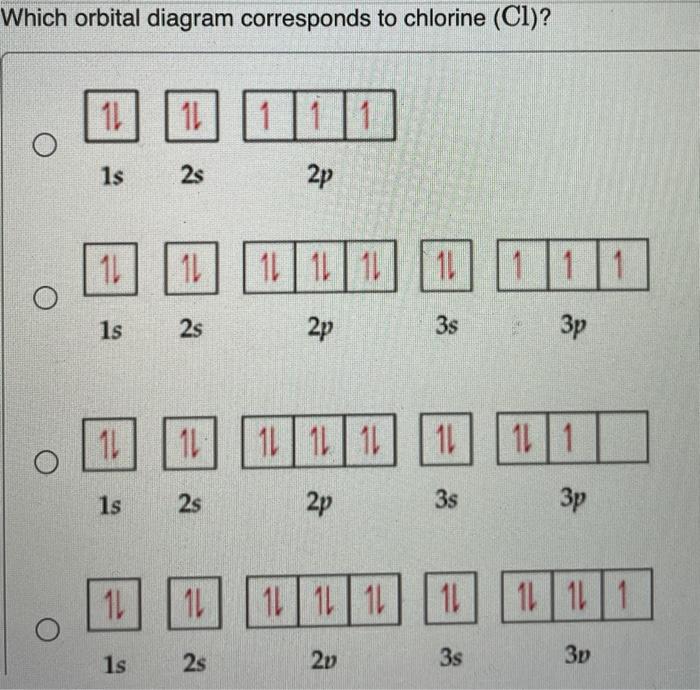
Jul 27, 2018 — Explanation: · 2s, only has 1 orbital, therefore 2s at most will hold max of 2 electrons . · 2p has 3 orbitals, and therefore can hold max of 3 x ...1 answer · full ground state electron configuration: 1s22s22p63s23p5 abbreviated: [Ne]3s23p5 Explanation: Chlorine has an atomic number of 17, which means it ...
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbital s, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. Oct 9, 2019 — The electronic configuration of a ground state chlorine is [Ne]3s23p5 (1s22s22p63s23p5).
Fluorine (F) Electron Configuration with Full Orbital Diagram. Fluorine electron configuration is 1s 2 2s 2 2p 5. The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine (F) and the orbital diagram is the main topic of this article.





![5 Steps]Electronic Configuration for Chlorine (Cl) in Just 5 ...](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjxKYyncD7ZlF3X7yzuV-ajYFNt3rACM9cT4rJeTMKS3HmKHUvHMn4EV4j_TFukqdAoW7_kena-triwvrGJCzxqTGJDzk2RveT0jMA7FmpjEPgbYultQO2d4tKCZytaoE0aTl6MCZ0taJk/s1600/20190115_195133.jpg)














0 Response to "37 orbital diagram for chlorine"
Post a Comment