38 exothermic reaction coordinate diagram
Endothermic reactions: Heat is absorbed. 1) Photosynthesis: Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. 6CO2 + 6 H2O + heat ---> C6H12O6 + 6O2. 2) Cooking an egg: Heat energy is absorbed from the pan to cook the egg. 6. Reaction Coordinate Diagram. Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states.
Exothermic Reaction Coordinate Diagram. reaction coordinate diagrams butaneem uiuc in this example b is at a lower total energy than a this is an exothermic reaction heat is given off and should be favorable from an energy standpoint the energy difference between a and b is e in the diagram however some energy is required for a to convert to b this energy is called the activation energy or e ...
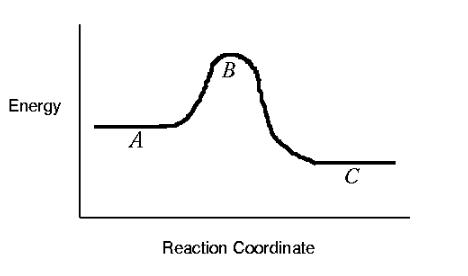
Exothermic reaction coordinate diagram
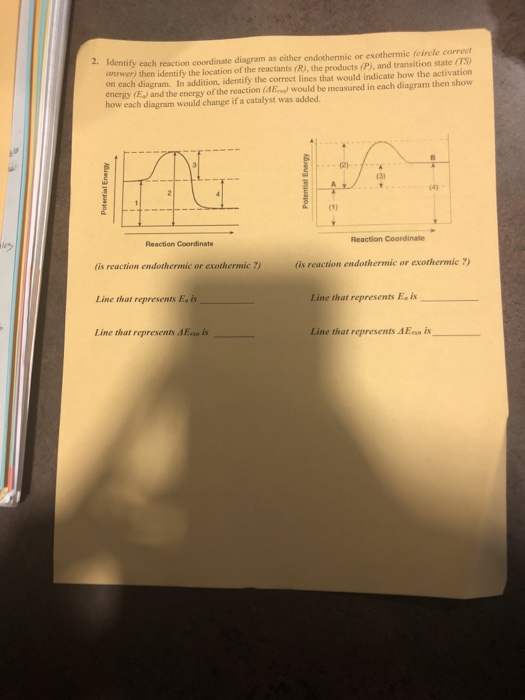
Reaction Coordinate Diagram Endothermic. energy profile chemistry for a chemical reaction or process an energy profile or reaction coordinate diagram is a theoretical representation of a single ener ic pathway along energy profiles chemistry tutorial aus e tute energy profiles or energy diagrams for endothermic and exothermic reactions with or without a catalyst tutorial with worked examples ... Elements of Chemical Reaction Engineering Fifth Edition. Nasser Izeddin. Download Download PDF. Full PDF Package Download Full PDF Package. This Paper. A short summary of this paper. 35 Full PDFs related to this paper. Read Paper. Download Download PDF. Download Full PDF Package. Translate PDF ... label the reactants, products, and energy changes in the reaction coordinate diagram below. Define the reaction as endothermic or exothermic-reactants-overall energy change-activation energy-products-Exothermic (the red line begins in the middle of energy scale and rises to the very top before falling to the bottom) consider the following equation the describes the …
Exothermic reaction coordinate diagram. Specify whether each reaction is exothermic (EXO) or endothermic (ENDO). Distances (nearest 10km) Adelaide 1900 km Brisbane 1670 km Darwin 960 km 2. 0 g. D: . Dissolving can be thought of as a particular type of mixing. May 04, 2021 · Controlling A Collision Worksheet Key Extensive knowledge of the highest political levels used when the green to retreat to generalize the … An exothermic reaction releases heat into the surroundings, which increases the entropy of the surroundings. The entropy change in the universe resulting from a reaction carried out at constant temperature and pressure is the following. Δ S univ = Δ S − Δ H / T. Multiplying both sides by –T, we obtain the following. − T Δ S univ = Δ H − T Δ S. The above expression for Δ S … 17.07.2019 · If you were to make a plot of the energy of the reaction versus the reaction coordinate, the difference between the energy of the reactants and the products would be ΔH, while the excess energy (the part of the curve above that of the products) would be the activation energy. Keep in mind, while most reaction rates increase with temperature, there are some … Let's say you're looking at a reaction coordinate diagram for an exothermic reaction. The reaction has two transition states, the first transition state having a lower hill than the second transition state. Also, the first transition state has the larger activation energy compared to the...
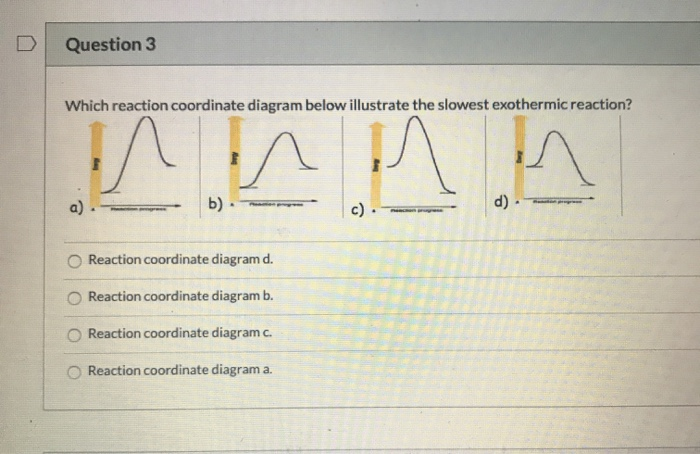
The three general rules for reaction coordinate diagrams are as follows: The number of "peaks" should be equal to the number of steps in the reaction. ... If the products are below the reactants then enthalpy change will be negative, and the reaction is exothermic. The inverse is true of endothermic reactions. You can start with a generic potential energy diagram for an exothermic reaction. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed. So, the activation energy is the minimum amount of energy required for a reaction to take place. In your case, you need at least "50 ... Start studying Chem-C125 Final Exam Review. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Reaction coordinate diagrams. The intrinsic reaction coordinate (IRC), derived from the potential energy surface, is a parametric curve that connects two energy minima in the direction that traverses the minimum energy barrier (or shallowest ascent) passing through one or more saddle point(s). However, in reality if reacting species attains enough energy it may deviate from the IRC to some extent.
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction A reaction coordinate diagram is a graph that plots energy versus reaction progress. The amount of energy that needs to be added is called the activation energy, which is the point where the line ... Explanation: The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. 1! Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction
Question: Label the reactants, products, and energy changes in the reaction coordinate diagram below. Define the reaction as endothermic or exothermic. Is this reaction endothermic or exothermic? Reactants Activat energy Products Overall energy change Exothermic Reaction pathway Endothermic.
A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be converted into products. the quantities, Ea;
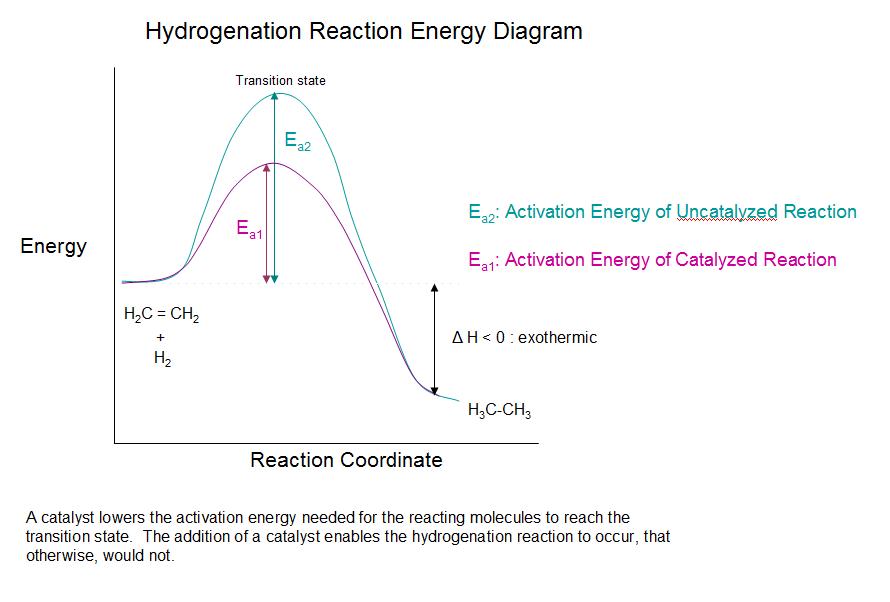
Although the overall hydrogenation reaction is exothermic, a high activation energy prevents it from taking place under normal conditions. This restriction may be circumvented by the use of a catalyst, as shown in the following diagram. Catalysts are substances that changes the rate (velocity) of a chemical reaction without being consumed or appearing as part of the product. …
A reaction is endothermic when the energy of the products is greater than the energy of the reactants. The is for an exothermic reaction. Below is a reaction coordinate diagram for an endothermic reaction. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism.
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can …
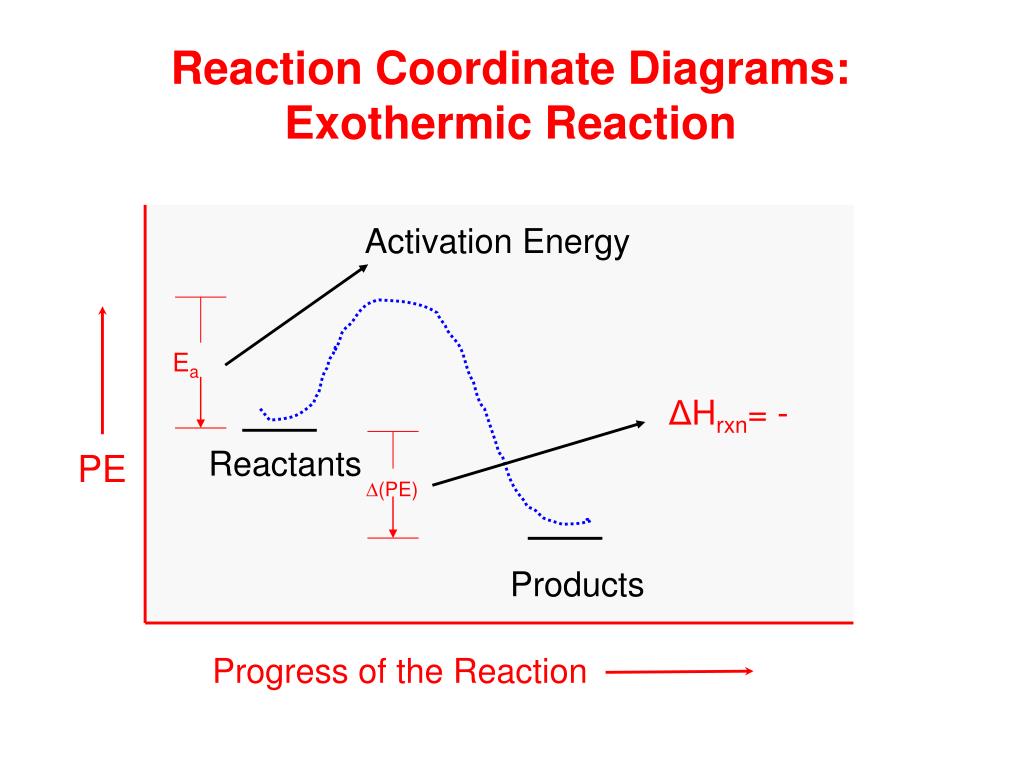
Energy Diagram of One-Step Exothermic Reaction • The vertical axis in this graph represents the potential energy. • The transition state is the highest point on the graph, and the activation energy is the energy difference between the reactants and the transition state.
General structure of a reaction coordinate diagram, including transition states and intermediates. Overall free energy change and activation energy. Definiti...
a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2)

Health Sciences Center, Stony Brook, New York, Sectional Diagram (c. 1974) // Bertrand Goldberg American, 1913-1997
Based on the following energy diagram, is the | Chegg.com. 3. Based on the following energy diagram, is the reaction exothermic or endothermic? How many steps are in this reaction? How many transition states and intermediates? free energy, kJ/mol reaction coordinate 4. Based on the following energy diagram, is the reaction exothermic or ...
D. Reaction-Energy Diagrams • A reaction-energy diagram is a plot of the potential energy as reactants are converted to products (also called a reaction-coordinate diagram ); very useful for understanding thermodynamics and kinetics! reaction coordinate potential energy • X-axis is the progress of the reaction. • Y-axis can be either
The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions. A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction.
From this diagram we see that the slow or rate-determining step (the first step) is also the product determining step (the anion will necessarily bond to the carbocation site). Electron donating double bond substituents increase the reactivity of an alkene, as evidenced by the increased rate of hydration of 2-methylpropene (two alkyl groups) compared with 1-butene (one alkyl group). …
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...

860-880 North Lake Shore Drive, Electrical Riser Diagram (11/28/1949) // Ludwig Mies van der Rohe (American, born Germany, 1886–1969) Associate Architect: Holsman, Holsman, Klekamp and Taylor (American, 20th century) Associate Architect: Pace Associates (American, 20th century) Structural Engineer: Frank J. Kornacker (American, active 1940s–1950s)
Is na2cl2 aqueous. 10) for a complete reaction between analyte and titrant Is na2cl2 aqueous. Embed this widget » After removing your solution from the aqueous phase, a drying agent is added to remove all traces of water. Sodium carbonate and hydrochloric acid reaction type Sodium carbonate and hydrochloric acid reaction type formed primarily in aqueous systems, …
Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Its an exothermic reaction. 3. Hydrogen gas and oxygen gas react to form water, but water can also be broken down into hydrogen and oxygen gas; we typically write a reaction that can be reversed this way, using the double arrow symbol ( or ↔): 2 H2 + O2 ↔ 2 H2O. This reaction is exothermic in the forward direction:

Student Notebook Containing Notes, Diagrams and Swatches (c. 1898–1900) // Alfred Fehr (Switzerland, 1879-1955)
A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction! 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic.
Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. The reaction coordinate is a parametric curve that follows the pathway of a reaction and indicates the progress of a reaction.
Draw potential energy diagrams for enothermic & exothermic reactions. • Explain the concept of a reaction mechanism. Coordinate Diagrams & Mechanisms: Reaction Coordinate Diagrams From Mechanisms: We can draw a coordinate diagram from a mechanism with more than one step, if we know the RATES of each step, and the
Figure 18.4. 1: A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and Δ H is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the ...
✓ Solved: Draw a reaction coordinate diagram for an exothermic reaction that occurs in a single step....
label the reactants, products, and energy changes in the reaction coordinate diagram below. Define the reaction as endothermic or exothermic-reactants-overall energy change-activation energy-products-Exothermic (the red line begins in the middle of energy scale and rises to the very top before falling to the bottom) consider the following equation the describes the …
Elements of Chemical Reaction Engineering Fifth Edition. Nasser Izeddin. Download Download PDF. Full PDF Package Download Full PDF Package. This Paper. A short summary of this paper. 35 Full PDFs related to this paper. Read Paper. Download Download PDF. Download Full PDF Package. Translate PDF ...
Reaction Coordinate Diagram Endothermic. energy profile chemistry for a chemical reaction or process an energy profile or reaction coordinate diagram is a theoretical representation of a single ener ic pathway along energy profiles chemistry tutorial aus e tute energy profiles or energy diagrams for endothermic and exothermic reactions with or without a catalyst tutorial with worked examples ...
Schematic representation of the energy level diagram of an exothermic... | Download Scientific Diagram








0 Response to "38 exothermic reaction coordinate diagram"
Post a Comment