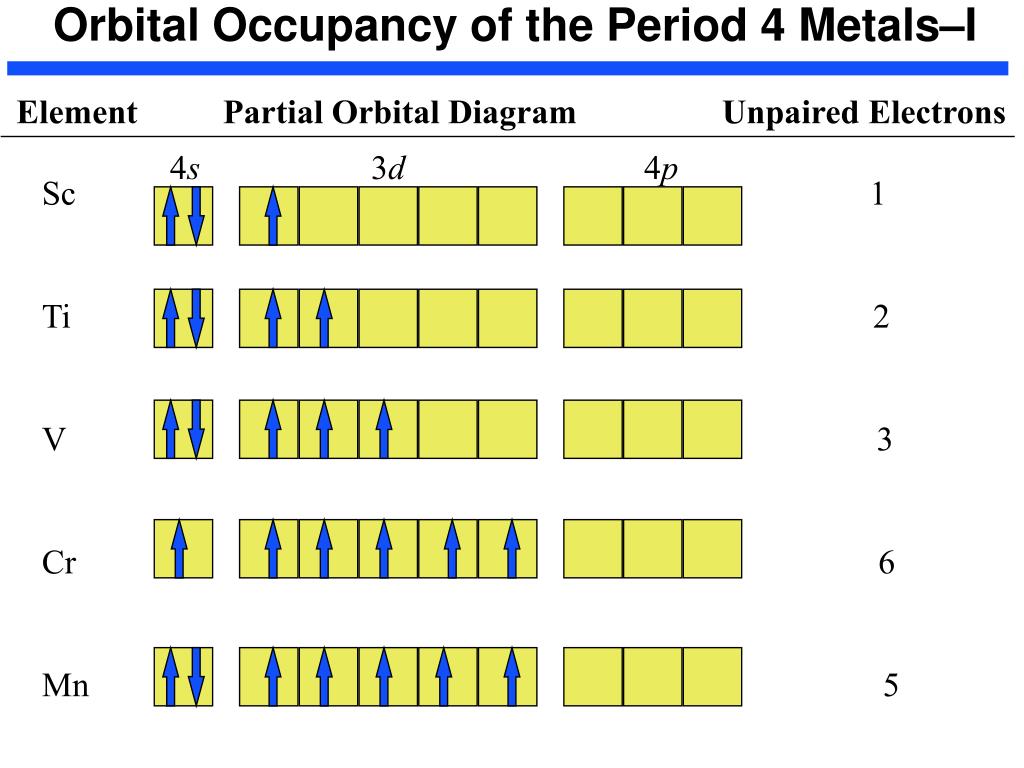
39 orbital diagram for sc
The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining two electrons. Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2. Note: I am also posting this to r/cscareerquestions Hi, I'm looking to plan for the start of my career, so I figured it was the right time to dive into this. I'm entering my final year of university in September (Studying Electrical & Mechanical Engineering), and they've given us a wide variety of options for the courses we can take (I didn't have any choices up until now), so I'm trying to pick the best combination of courses to pursue a career in Robotics/Control. I should note that I eng...
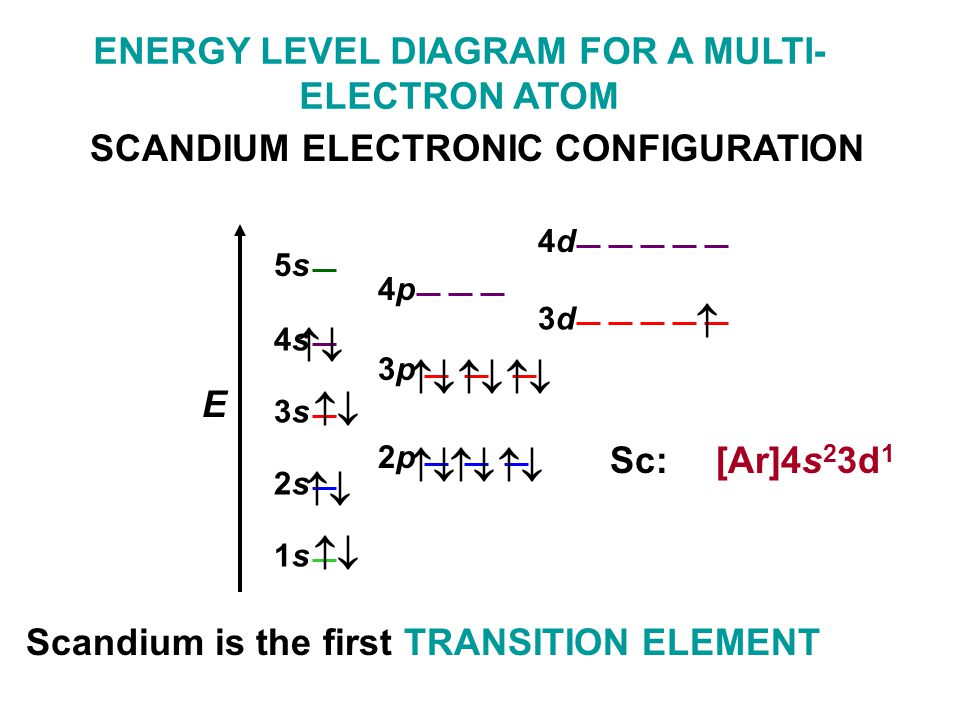
An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 ...
Orbital diagram for sc
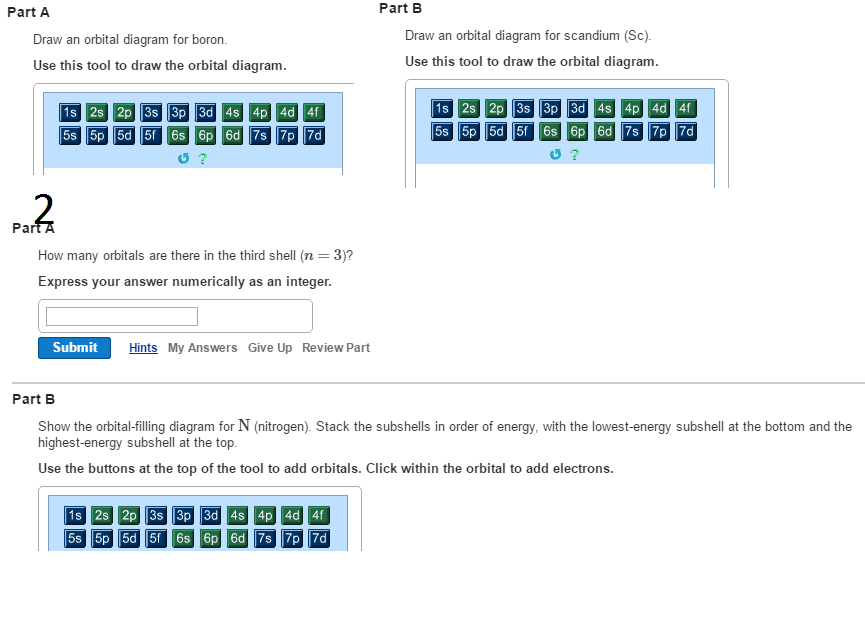
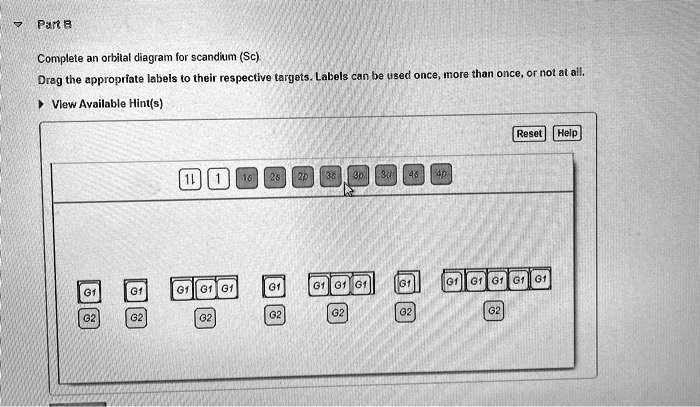
Section 1.6 - 4 • A general term symbol that uniquely describes a specific electronic configuration looks like this: (2S+1)L J where 2S + 1 is the spin multiplicity (and S is the total spin angular momentum.) L is the total orbital angular momentum J is the total angular momentum (spin + orbital) S = 0 → "Singlet" S = ½ → "Doublet" S = 1 → "Triplet" etc. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron ... Answer to Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1.
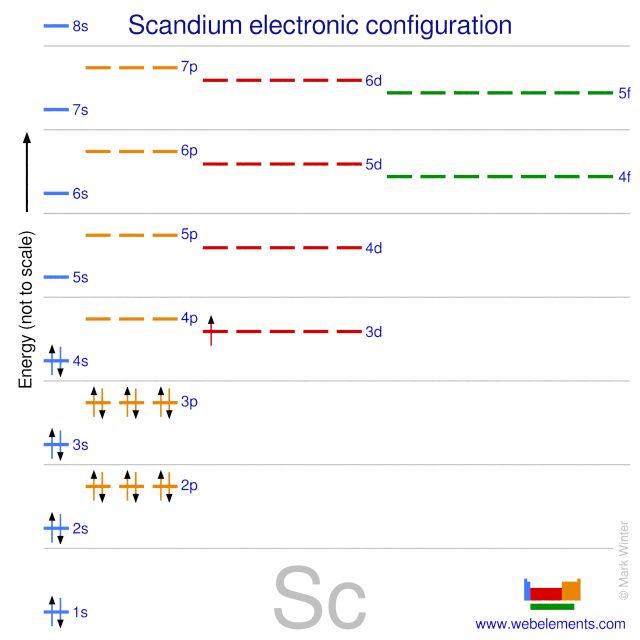
Orbital diagram for sc. Greetings, Commanders! o7 In [Elite: Dangerous - Beyond \(3.3+\)](http://elite-dangerous.wikia.com/wiki/Elite_Dangerous:_Beyond), we now need (would like?) to solve the problem of efficient planet mapping. Simply put, we need to use a minimum number of probes to cover at least 90% of the surface of a given orbital body by the spherical caps generated by the probes’ scan range. Fortunately, FDev has given us an idea of the number of probes needed in the “Efficiency Target”, and this will guid... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. Answer to Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. 3d. 4p. Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation). 1. scandium. ↑↓. ↑↓. ↑↓ ↑↓ ↑↓. ↑↓. Contrary to what you may have seen, for Sc and the remaining elements ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
Orbital symmetry correlation diagrams 3. Huckel-Mobius theory or Perturbation Molecular Orbital (PMO) 20S.Ravikumar M.Sc 21. 1. Frontier Molecular Orbital (FMO) approach The mechanism of electrocyclic reactions can be understood in terms of frontier molecular orbital (FMO) theory and the outcome of reactions can be predicted using the Woodward ... An orbital diagram is the representation of electrons present in different orbitals. There are four orbitals, s-orbital, p-orbital, d-orbital, and f-orbital.1 answer · Top answer: Orbital diagram of Scandium The atomic number of Sc is 21. The electronic configuration of Sc is... Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. 1s: 2 arrows 2s: 2 arrows 2p: 2 arrows in all 3 orbitals 3s: 2 arrows 3p: 2 arrows in all 3 orbitals 4s: 2 arrows • In the orbital approximation, ... The above diagram roughly depicts the relative energy difference between these three ways of filling 2 electrons into the three p orbitals. ... (Sc) provide a really good example for this argument. Sc [Ar] 3d1 4s2 Sc+ [Ar] 3d1 4s1
Science; Chemistry; Chemistry questions and answers; Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. 1s, 2s, 2p, 3s, 3p, 3d, 4s ... Complete an orbital diagram for scandium (Sc). you must draw an electon in boxes diagram the full configuration is: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 s orbital has 1 box (squares) p orbital has 3 boxes d orbital has 5 boxes in each box fill it with arrows (up and down Scandium is a chemical element with the symbol Sc and atomic number 21. For example ... Chemistry questions and answers. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Question: Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once ... A. Kapur, S. Kapur, and P. Maes, "AlterEgo: A Personalized Wearable Silent Speech Interface." 23rd International Conference on Intelligent User Interfaces (IUI 2018), pp 43-53, March 5, 2018. https://dam-prod.media.mit.edu/x/2018/03/23/p43-kapur_BRjFwE6.pdf >we propose a peripheral nerve interface by taking measurements from the facial and neck area, which allows for silent speech signals to be distilled without being accompanied by electrical noise from the frontal lobe of the cerebral cor...
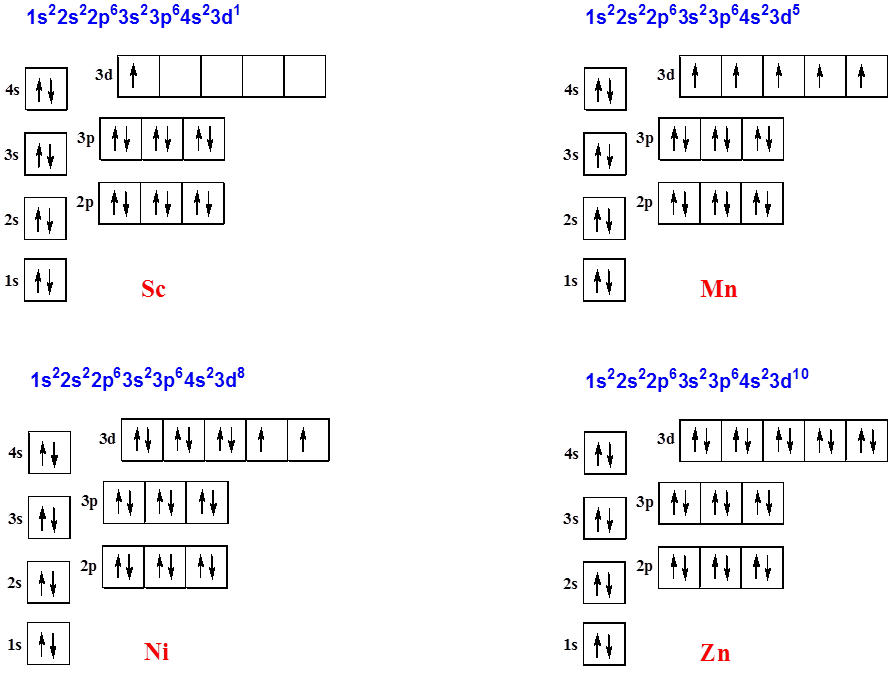
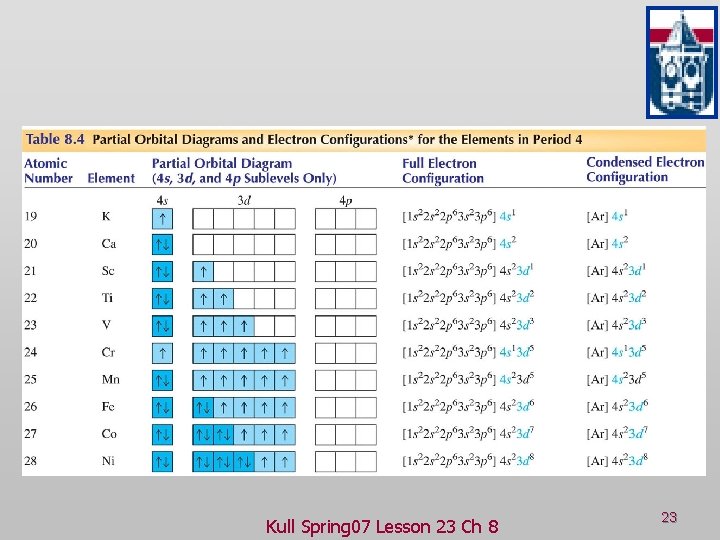
After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s.
Posted this on /r/HomeworkHelp but gona post here as well, if some1 got freetime feel free to double check my work below! Basically we did a diels-alder lab where we reacted maleic anhydride and furan, measure melting point and decide from melting point using literature melting point if the product is endo or exo and therefore if its under kinetic or themodynamic control. **Heres the overall reaction:** https://prnt.sc/pjd50y Anyways its asking me to identify of my product is endo or exo. Thi...
Jul 29, 2021 — draw orbital diagram scandium sc. Discover The Secrets Of Drawing Realistic Pencil Portraits. This will help you to achieve mastery in a ...
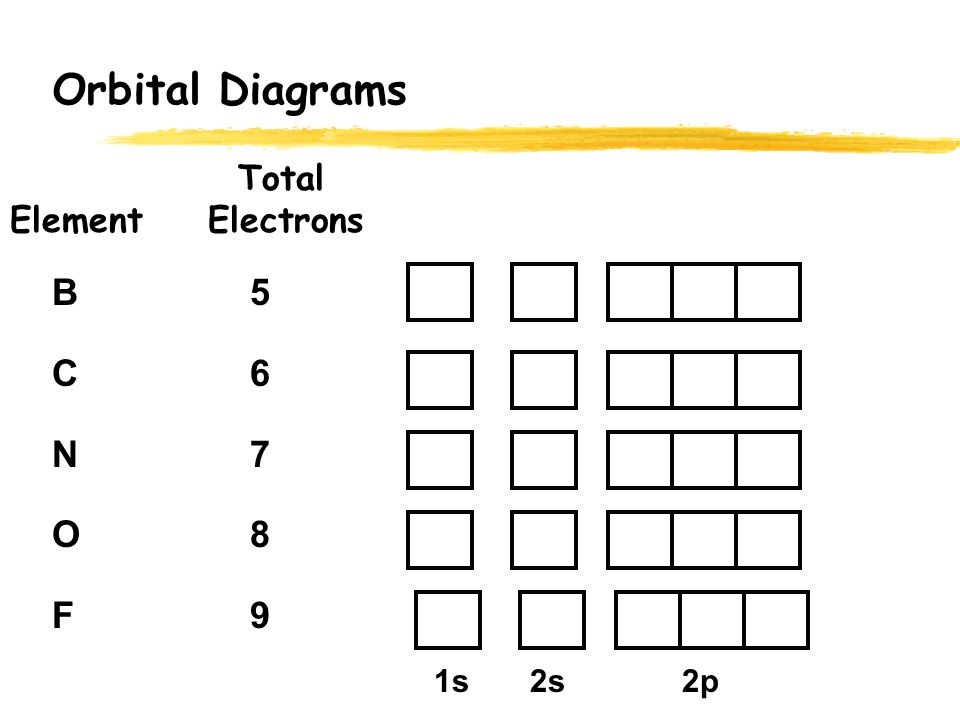
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
Basically we did a diels-alder lab where we reacted maleic anhydride and furan, measure melting point and decide from melting point using literature melting point if the product is endo or exo and therefore if its under kinetic or themodynamic control. **Heres the overall reaction:** https://prnt.sc/pjd50y Anyways its asking me to identify of my product is endo or exo. This one is easy because I can compare the melting point to the literature melting point and so I know I get the exo product. ...
Feb 1, 2021 — Full electron configuration for Sc can be represented as: [Ar] 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹ 4s². What is The Electron Configuration of Scandium.
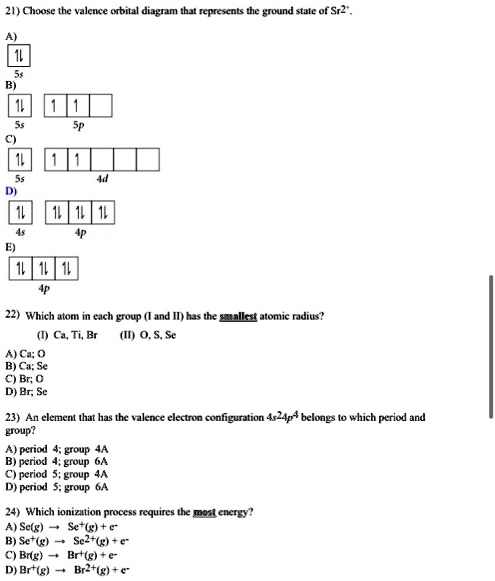
Problem: Part B. Complete an orbital diagram for scandium (Sc)Draw orbital diagrams, and use them to derive electron configurationsTo understand how to draw orbital diagrams, and how they are used to write electron configurations.The electron configuration of an element is the arrangment of its electrons in their atomic orbitals. Electron configurations can be used to predict most of the ...
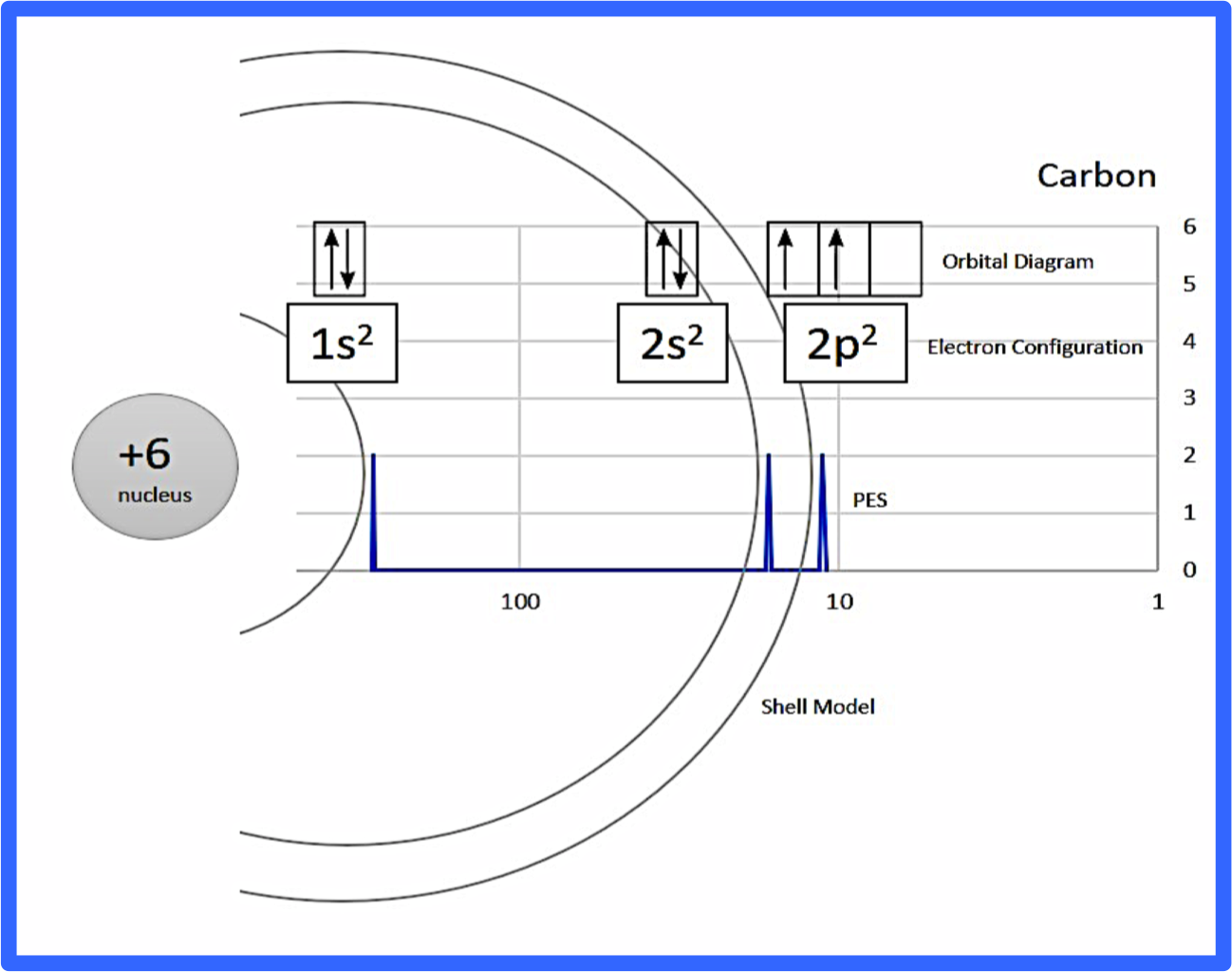
Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy.
Question: Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. This problem has been solved! See the answer ...
Answer (1 of 10): Sc -at. no. is 21 e.c.-1s2 2s2 2p6 3s2 3p6 4s2 3d1 Energy of 4s is lesser energy than that of 3d .According to Aufbau principle lower energy orbitals fills first.So,4s comes before 3d.
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. wiringall.com!
I was doing some research on Ni and Pd/Pt in the Suzuki reaction when I kind of digressed to look at trends in the first ionisation energies of the transition metals. I plotted a relatively crude graph comparing the actual ionisation energies of the transition metals with the 'ideal' values (just drawing a straight line between Sc and Zn) [Link](https://imgur.com/a/nmjpi). Looking at the graph, I'm able to explain why V/Co/Ni have IEs well below the line, because of the exchange energy they ga...
Period: 1930.72 days ||Submissions|Comments| :-:|--:|--: __Total__|999|49784 __Rate (per day)__|0.52|25.78 __Unique Redditors__|262|8712 __Combined Score__|624610|573056 --- ###Top Submitters' Top Submissions 1. 48694 points, 71 submissions: /u/__hrga__ 1. [French frigate Hermione next to a FREMM frigate [1913 × 1276]](https://i.imgur.com/Q0wbntN.jpg) (1529 points, [72 comments](/comments/av086i)) 1. [Hatsuyuki-class destroyers [1200 × 675]](https://i.imgur.com/7zKcs1l.jpg) (1064 points, [...
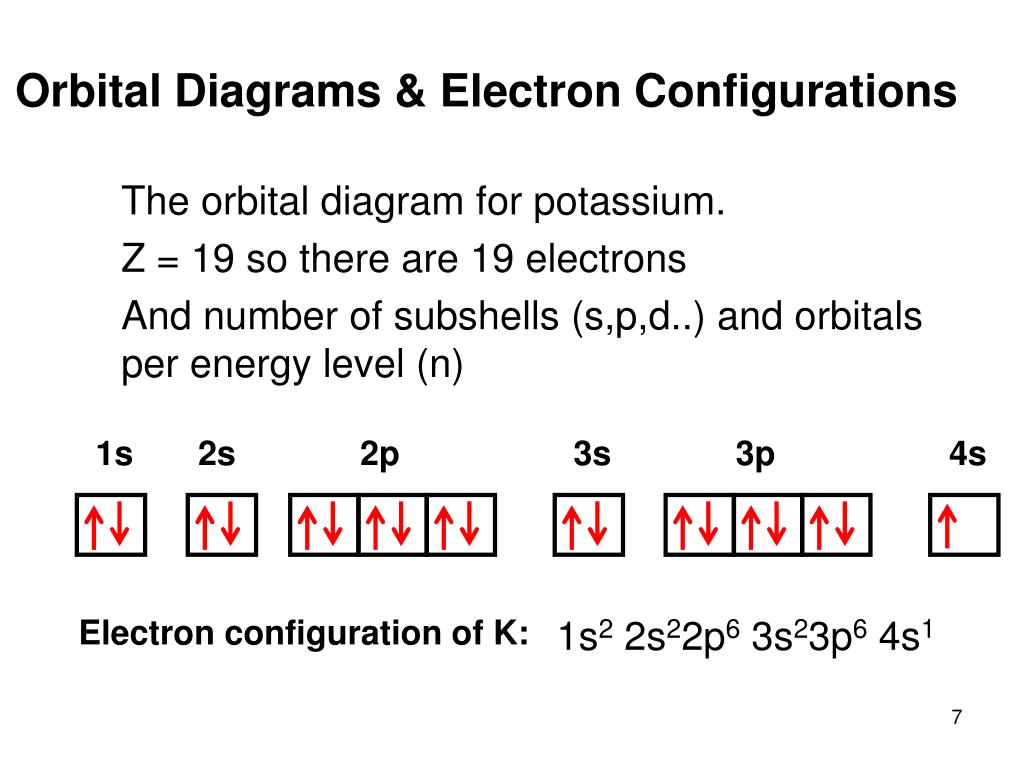
The s orbital holds a maximum of 2 electrons. The p orbital can hold 6. The d orbital can hold 10. The f orbital can hold 14 electrons. But, the orbitals overlap. The Madelung rule gives the order: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Orbital diagram for Sc. Wiki User. ∙ 2011-01-11 00:43:43. Study now. See Answer. Best Answer. Copy. the electronic configuration for sc is 1s2 2s2 2p6 3s2 3p6 4s2 3d1. Wiki User. ∙ 2011-01-11 ...
An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ...
Note: I am also posting this to r/robotics Hi, I'm looking to plan for the start of my career, so I figured it was the right time to dive into this. I'm entering my final year of university in September (Studying Electrical & Mechanical Engineering), and they've given us a wide variety of options for the courses we can take (I didn't have any choices up until now), so I'm trying to pick the best combination of courses to pursue a career in Robotics/Control. I should note that I engage in a...
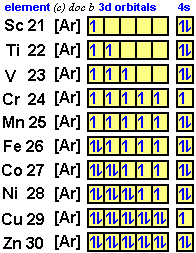
Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc ...
Scandium (Sc) has an atomic mass of 21. Find out about its chemical ... Electron Configuration, [Ar] 3d1 4s2. 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Orbital Diagram.
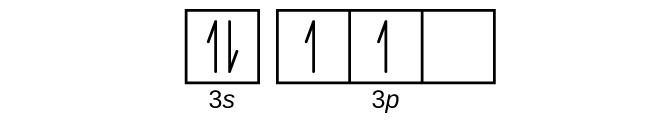
For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: ... Write a set of quantum numbers for each of the electrons with an n of 3 in a Sc atom.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Draw a molecular orbital diagram and determine the bond order expected for the molecule B. 2. For full credit on MO diagrams, • label increasing energy with an arrow next to the diagram. • pay attention to whether the question asks for valence electrons or all electrons. • for any bonding orbital drawn, include the corresponding anti ...
So I'm making this post for a few reasons. Personally, I want all of this off of my laptop and in a place I can easily get to on my phone to argue with people about climate change. The reason I'm posting it here is because I want Steven to have enough information to confidently confront climate change deniers and do the factual equivalent of a curb stomp from orbit, preferably on stream for my entertainment. Let's get riiiiiiiiiiiiiiight INTO THE MEMES! #Evidence of Climate Change Here is ev...
Electron configuration and orbital diagram for scandium ... , Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 . So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. This is a memory device to remember the order of orbitals for the first two quantum numbers.
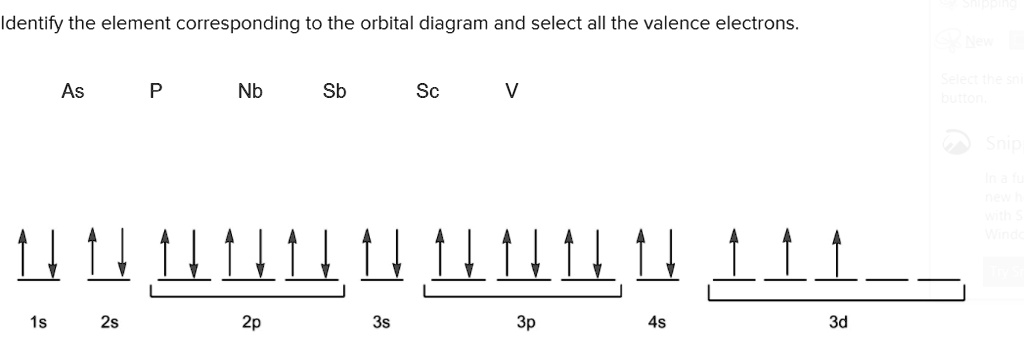
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Answer to Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1.
Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron ...
Section 1.6 - 4 • A general term symbol that uniquely describes a specific electronic configuration looks like this: (2S+1)L J where 2S + 1 is the spin multiplicity (and S is the total spin angular momentum.) L is the total orbital angular momentum J is the total angular momentum (spin + orbital) S = 0 → "Singlet" S = ½ → "Doublet" S = 1 → "Triplet" etc.




















0 Response to "39 orbital diagram for sc"
Post a Comment