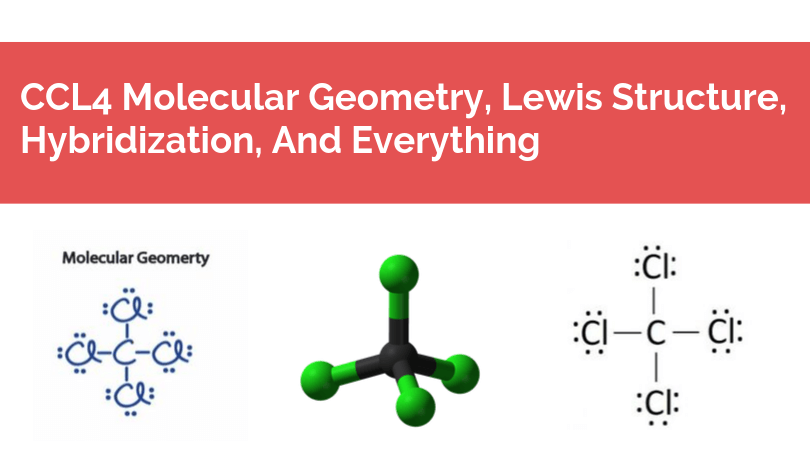
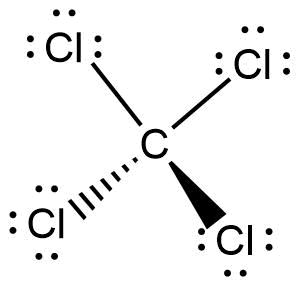
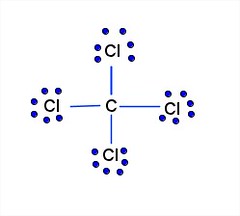
40 lewis diagram for ccl4
Thus to understand the Lewis structure in-depth lets go step by step in understanding the concept. First of all, to remind you Lewiss structure is a pictorial representation of different bonds and lone pair of electrons between two or more atoms of a compound. Step 1: The foremost step of creating a Lewis structure is finding the valence electrons. b. Lewis structures with large formal charges (e.g., +2,+3 and/or -2,-3) are preferred. c. The preferred Lewis structure is one in which positive formal charges are on the most electronegative atoms. 17. For which of these species does the best Lewis structure have two or more equivalent resonance structures? a. HCO2- b. SCN- c. CNO- d ...
Is Benzene Polar Or Non Polar Quora. Benzene, c6h6, is a hydrocarbon found in crude oil, and a major component of gasoline. it's used to make synthetic fibers, detergents and even drugs. you can derive benzoic acid, chemical structure c6h5cooh, from benzene by uniting of the water insoluble benzene molecule with a carboxylic acid group, ( cooh).

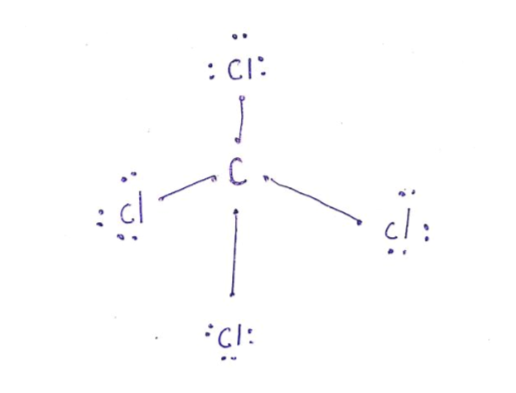
Lewis diagram for ccl4
CH2Cl2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. Dichloromethane or methylene chloride, with the chemical formula CH2Cl2, is a colorless, volatile liquid with a boiling point of 39.6 °C. and a melting point of -96.7 °C. It is widely used as a solvent in chemistry laboratories. It is polar because of the presence of ... NH3 is a polar molecule because, in the NH3 molecule, it has three dipoles because of three bonds and these dipoles do not cancel out each other. They form a net dipole moment. In Ammonia molecules three atoms of hydrogen form a covalent bond by sharing 3 electrons of nitrogen and hydrogen atoms leaving behind one lone pair on the nitrogen atom. 11+ Ccl4 Lewis Structure. The lewis structure for ccl4 is a commonly tested lewis. To figure out lewis structure, do this: carbon tetrachloride - Wikidata from upload.wikimedia.org We could write it as well as a structural formula that would look like this right here. The lewis structure indicates that each…
Lewis diagram for ccl4. Lewis StructuresWe also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions, For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding ... Chloroform, or trichloromethane, is an organic compound with formula C H Cl 3. It is a colorless, strong-smelling, dense liquid that is produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. H2CO Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. It is an organic compound with the molecular formula of H2CO and is classified as an aldehyde. Aldehydes are chemicals having the functional group -HCO- in their molecules and formaldehyde is the lowest member of this group with a single carbon atom. Geometrical Structure: The geometrical shape of an atom is an important parameter to check its polarity. The molecule that is symmetric in shape is nonpolar whereas those whose shape is asymmetric (mainly because of lone pair) are polar in nature. Properties of NCl3. It exists as a yellow oily liquid at room temperature.
Benzene is an organic chemical compound with the molecular formula C 6 H 6.The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Diagrama De Lewis Ch4 ch4 lewis dot diagram structure draw molecular estructura methane cross ã ã ã ã Electron formula î CH4î svg Wikibooks ch4 methane punkte svg lewis structure chemistry valence electrons ch wikipedia commons structures each carbon file wikimedia molecule octet draw dot methane ch4 lewis diagram electron structure ... A Lewis structure is a model thatSbcl5 lewis dot suggestions (Click to sort alphabetically). Sbcl5 lewis dot stats.. that obey the octet rule it is possible to draw electron-dot, or Lewis, structures.and I-(b) Cs + and SO 4 2-, (c) Sr 2+ and N 3-(d) Al 3+ and S 2-9.18 Write theWhat do the dots represent in a Lewis dot diagram? Carbon monoxide exemplifies a Lewis structure with formal charges: To obtain the oxidation states, the formal charges are summed with the bond-order value taken positively at the carbon and negatively at the oxygen. Applied to molecular ions, this algorithm considers the actual location of the formal (ionic) charge, as drawn in the Lewis structure.
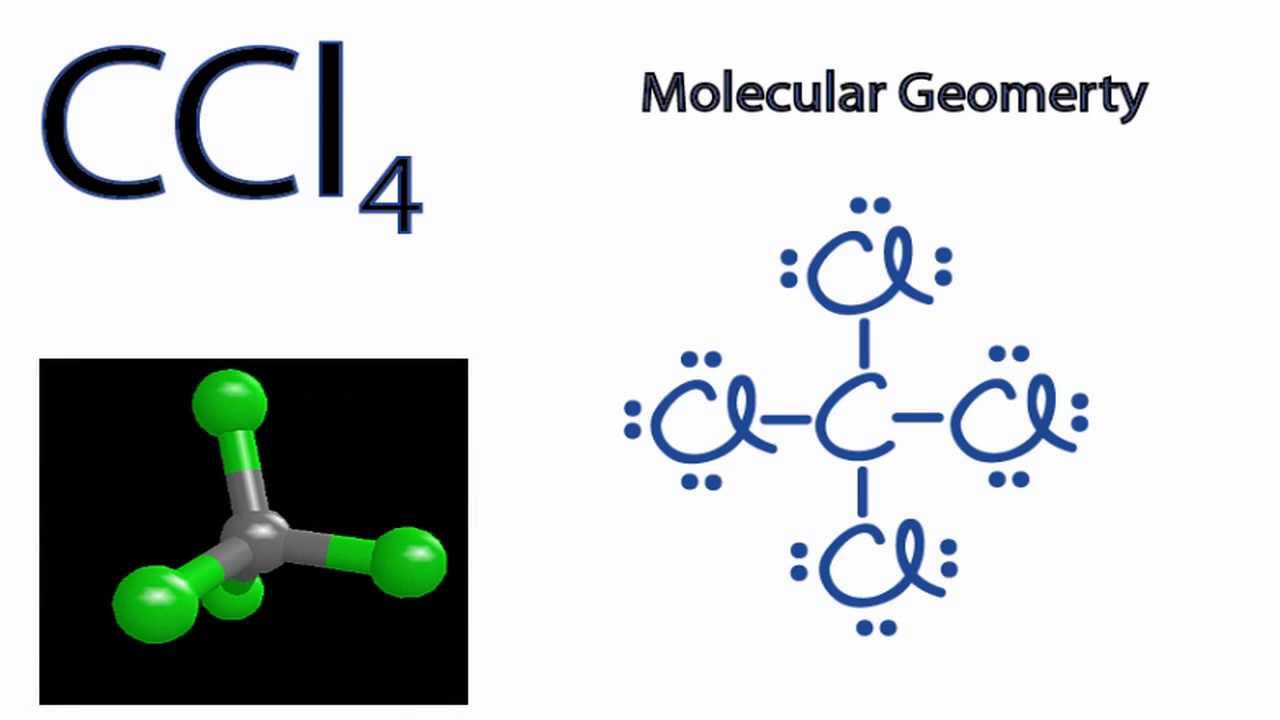
Examples of such molecules are CO2, O2, PCl5, etc. Check out the reason for the non-polarity of CCl4. Note: It may be possible to have the existence of a polar bond within a nonpolar molecule. This is because the polarity of such bonds gets canceled with each other due to the symmetrical geometrical structure of the molecule. Carbon Tetrachloride or CCl4 is a symmetrical molecule with four chlorine atoms attached to a central carbon atom. It has a tetrahedral geometry. Owing to the high electron affinity and small size of carbon and chlorine atom it forms a covalent C-Cl bond. The bond is a polar covalent bond due to the electronegativity difference. After lewis structure, there is a need of understanding its molecular geometry and hybridization of the central atom, Beryllium. The molecular orbital (MO) theory will be used to understand the MO diagram of beryllium chloride. BeCl2 Lewis Structure. The electrons present in the outermost shell of an atom are shown in the Lewis structure of any ... Lewis Structure does give us a viewpoint on the nature of bonds and formal charge concepts but it has its drawbacks. To have a clearer idea of a given molecule, we need to also have in-depth knowledge about the 3-dimensional nature. This is known as molecular geometry which tells us about the molecular shape.
The Lewis structure is a dot diagram to determine how many lone valence electrons are present and absent within an atom. Moreover, it is easy to figure out which bond has been for med between the atoms of a molecule, with the help of this diagram. Lewis dot diagram for o3.
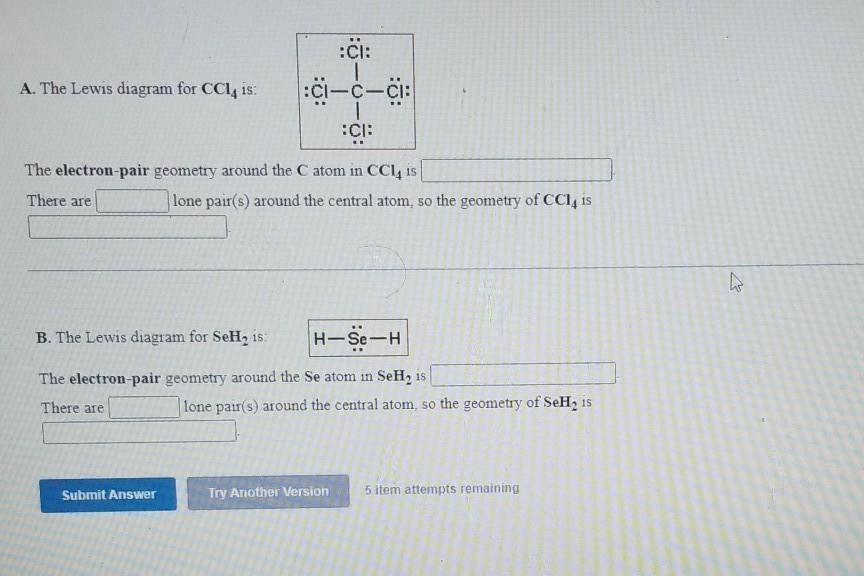
Lab 6 Molecular Geometry CHEM101L Student Name: Click here to enter text. Access Code (located on the lid of your lab kit): Pre-Lab Questions: 1. Describe the difference between valence and core electrons. 2. What is the octet rule and which elements obey the octet rule? 3. How does VSEPR affect the shape of molecules? […]
11+ Ccl4 Lewis Structure. The lewis structure for ccl4 is a commonly tested lewis. To figure out lewis structure, do this: carbon tetrachloride - Wikidata from upload.wikimedia.org We could write it as well as a structural formula that would look like this right here. The lewis structure indicates that each…
NH3 is a polar molecule because, in the NH3 molecule, it has three dipoles because of three bonds and these dipoles do not cancel out each other. They form a net dipole moment. In Ammonia molecules three atoms of hydrogen form a covalent bond by sharing 3 electrons of nitrogen and hydrogen atoms leaving behind one lone pair on the nitrogen atom.
CH2Cl2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. Dichloromethane or methylene chloride, with the chemical formula CH2Cl2, is a colorless, volatile liquid with a boiling point of 39.6 °C. and a melting point of -96.7 °C. It is widely used as a solvent in chemistry laboratories. It is polar because of the presence of ...
























0 Response to "40 lewis diagram for ccl4"
Post a Comment