40 orbital filling diagram for calcium
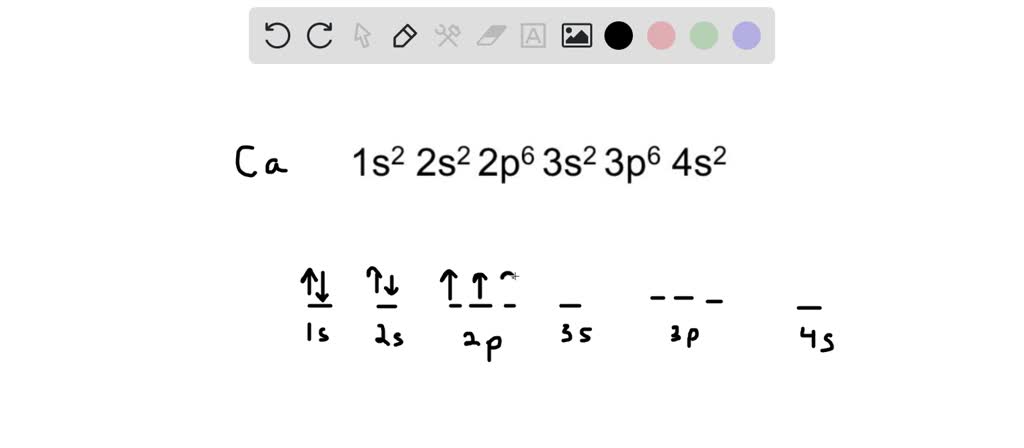
Calcium Orbital Filling Diagram 04.03.2019 3 Comments In order to write the Calcium electron configuration we first need to know the we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. The atomic number of calcium is This means that in a neutral calcium atom, there are 20 protons in its nucleus.
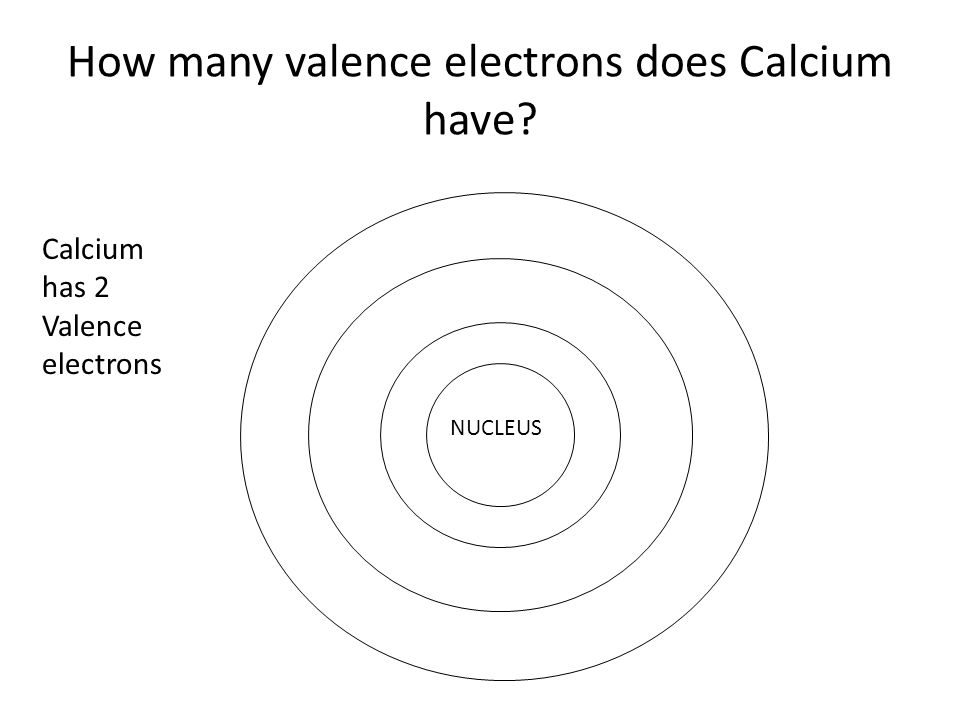
Electronic configuration of the Calcium atom. Valence electrons. Orbital diagram.Missing: filling | Must include: filling
In a neutral atom, the number of protons is equal to the number of electrons. So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s22s22p63s23p64s1. The noble gas notation is [Ar]4s1. The following orbital diagram shows the increase in energy from one energy sublevel to the next ...
Orbital filling diagram for calcium
Figure 2. An Aufbau diagram that illustrates the (n+l) rule. The (n + l) rule is a remarkably clever and useful tool. It correctly predicts the order of orbital energies through element 20 (calcium).
Here is an example of orbital configuration for Hydrogen, Helium and Carbon. The simplest atom hydrogen has 1 electron. It will go into the 1s orbital with a spin in either direction. We can represent this with either an orbital filling diagram or an electron configuration. The next atom is helium. It has 2 electrons.
August 15, 2020 - Note that the filling of electrons in each orbital (px, py and pz) is arbitrary as long as the electrons are singly filled before having two electrons occupy the same orbital. (a)This diagram represents the correct filling of electrons for the nitrogen atom. (b) This diagramrepresents the incorrect ...
Orbital filling diagram for calcium.
1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ...
January 22, 2021 - Calcium Electron Configuration (Ca) with Calcium Orbital Diagram and its symbol are present here. More infomation about the Ca given here.
Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.
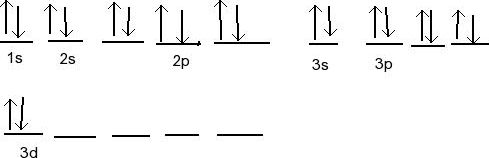
Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of ...
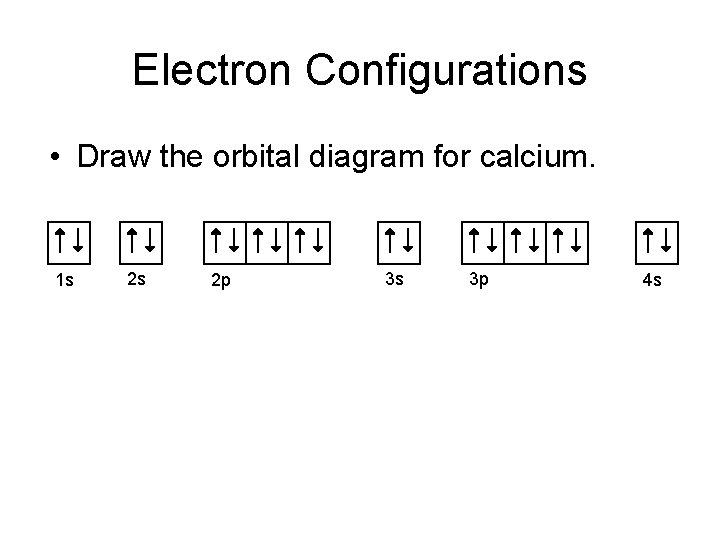
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
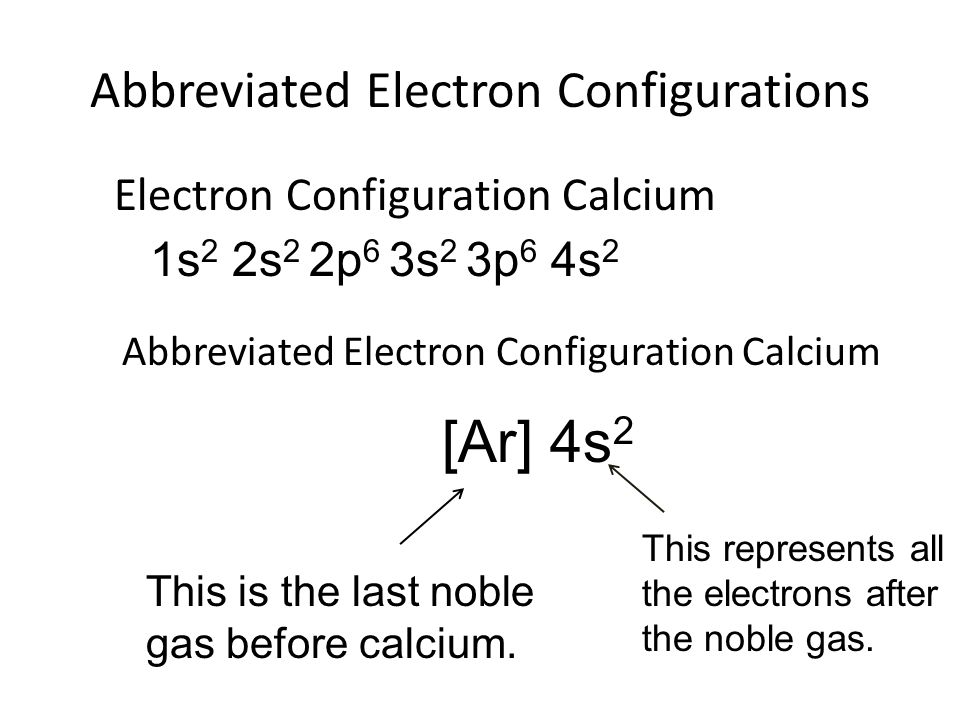
Understanding the above rules and diagrams will allow you to determine the electron configuration of almost any atom or ion. How to Write the Electron Configuration of an Isolated Atom[edit | edit source] Electron-configuration notation is relatively straightforward. An isolated Calcium atom 20Ca, ...
October 31, 2018 - Answer (1 of 4): The atomic number of calcium is 20. This means that in a neutral calcium atom, there are 20 protons in its nucleus. A neutral calcium atom also has 20 electrons. The electron configuration of a neutral calcium atom is : 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 . A calcium 2+ ion has lost i...
Draw the orbital filling diagram for carbon and write its electron configuration. Step 1: List the known quantities and plan the problem. Known. atomic number of carbon, Z = 6; Use the sublevel energy ordering illustrated above (Figure above) to draw an orbital filling diagram with a total of six electrons. Follow Hund's rule.
The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals. They are shown at a slightly higher level than the 4s - and so it is the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals.
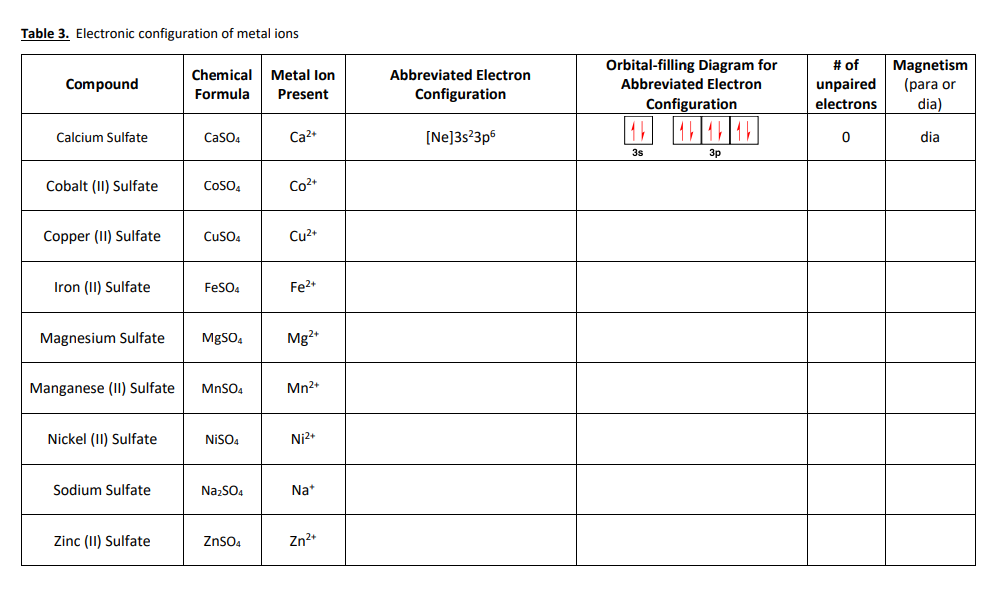
Table 3. Electronic configuration of metal ions Compound Chemical Metal lon Formula Present Abbreviated Electron Configuration Orbital-filling Diagram for Abbreviated Electron Configuration # of unpaired electrons Magnetism (para or dia) Calcium Sulfate CaSO4 Ca2 [Ne]3s23p6 11111111 0 dia 3s 3p Cobalt (11) Sulfate CoSO4 Co2+ Copper (11) Sulfate ...
Orbital diagrams are pictorial representations of the arrangement of Rather than filling the orbitals one-by-one, it is easier to note that calcium is in the s- block. When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. Here I use the electron.
A step-by-step description of how to write the electron configuration for Calcium (Ca). In order to write the Ca electron configuration we first need to know...
Under the orbital approximation, we let each electron occupy an orbital the atomic number of calcium is 20. And the electron configuration is the standard notation used to describe the electronic structure of an atom. Calcium Orbital Filling Diagram Source: i.ytimg.com. What Is The Ground State Electron Configuration Of Calcium …. You have to ...
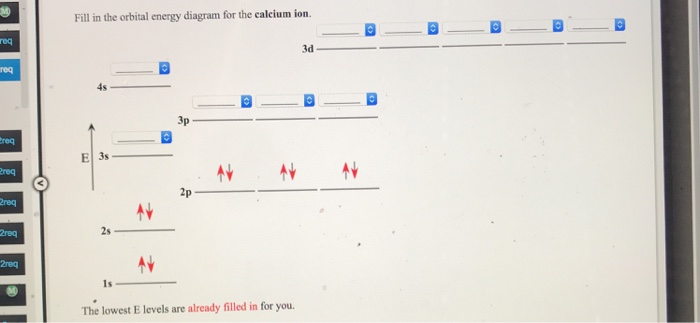
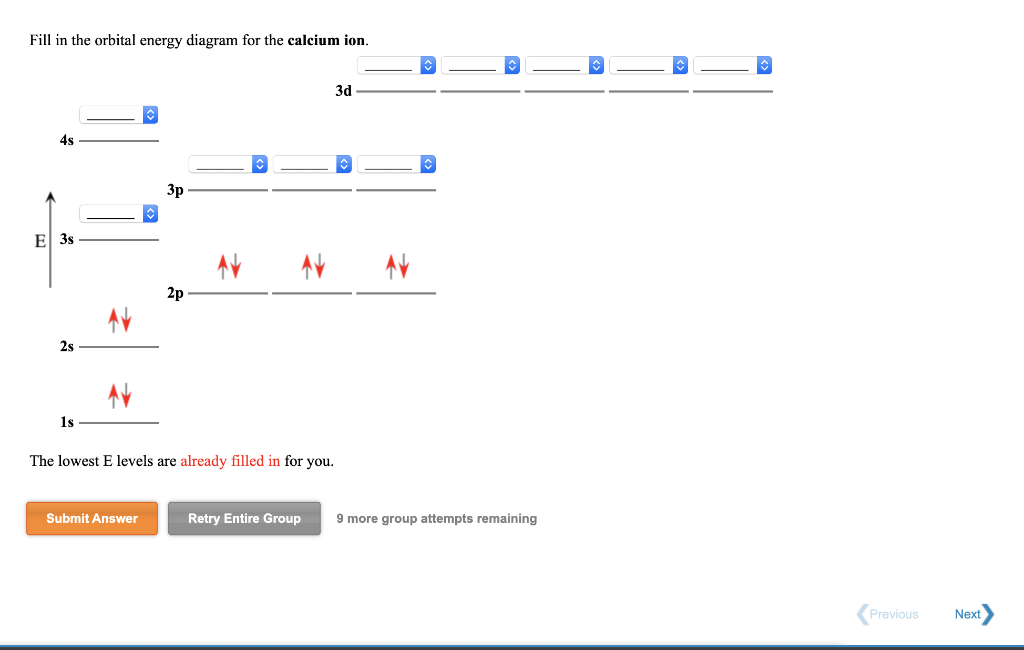
Is this the correct atomic orbital diagram for a calcium (2+) ion? Please indicate "true" or "false" accordingly on your Google quiz form.
What is the orbital diagram for calcium? Orbital Diagrams: Orbital diagrams are pictorial representations of the arrangement of electrons within the orbitals in an atom or molecule. The orbitals...
Problem: Part AWrite the electron configuration for each ion.Co2+N3-Ca2+Express your answers in condensed form separated by commas. Part BDraw the orbital diagram for ion Co2+.Part CDraw the orbital diagram for ion N3-?Part DDraw the orbital diagram for ion Ca2+.
Draw an energy level orbital filling diagram for just the valence electrons in pd is this ion paramagnetic or diamagnetic consider the ground state of arsenic as a how many valence electrons 30481
The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 3 or Figure 4). Thus, the electron configuration and orbital diagram of ...
Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1.
In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
Which of the following is the correct orbital diagram for a nitrogen (n) atom_. Write out the orbital box diagram and the condensed electron configuration for Silicon: 14si=1s^2, 2s^2, 2p^6, 3s^2, 3p^2 / condensed - E:C: = [Ne] 3s^2, 3p^2 9. The two dots represent the n nuclei.
Write out the full electron configuration of calcium. Draw the full orbital diagram of calcium.
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
What does an orbital diagram look like? In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal energy level and sublevel. Electrons are indicated by arrows inside the circles.
August 30, 2018 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
March 11, 2016 - Quora is a place to gain and share knowledge. It's a platform to ask questions and connect with people who contribute unique insights and quality answers.
Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level. The oddity is the position of the 3d orbitals.
The Order of Filling Orbitals. Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. Where there is a choice between orbitals of equal energy, they fill the orbitals singly as far as possible. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level.
How many unpaired electrons does calcium have? You may need to draw the orbital filling diagram for the last sublevel that contains electrons. 0 The number of unpaired electrons in an atom can be determined from the orbital filling diagrams and correctly applying Hund's rule.
Hund©s Rule & Orbital Filling Diagram Complete the orbital diagram for each element. 2) calcium 1s 2s 4s 3s 3d 2p 4p 3p 1) sodium 1s 2s 4s 3s 3d 2p 4p 3p 3) nickel 1s 2s 4s 3s 3d 2p 4p 3p 4) silicon 1s 2s 4s 3s 3d 2p 4p 3p 5) iron 6) copper 1s 2s 4s 3s 3d 2p 4p Answer key 3p 2s2 2p6 3s2 3p6 4s2 1s 2s 4s 3s 2p 4p 3p 3d. Created Date:
In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
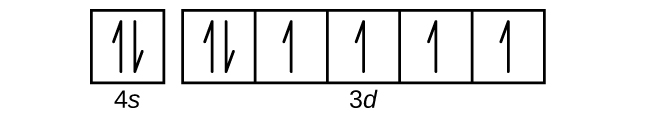
Calcium (Ca) ; Thermal Conductivity · 200.0 W·m·K ; Oxidation States, 2 ; Electrons Per Shell, 2 8 8 2 ; Electron Configuration, [Ar] 4s ; Orbital Diagram. 1s. ↿⇂.
The orbital filling diagram of lithium. A special type of notation is used to show an atoms electron configuration. Pure metal is produced by replacing the calcium in lime calcium carbonate CaCO3 with aluminium in hot low pressure retorts. If you dont have a chart you can still find the electron configuration.
Our calcium page has over 250 facts that span 115 different quantities. Each entry has a full citation identifying its source. Areas covered include atomic structure, physical properties, atomic interaction, thermodynamics, identification, atomic size, crystal structure, history, abundances, ...
An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
Chem4Kids.com! Calcium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
Orbital Diagram For Barium. The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for Barium. Barium is an alkaline earth metal. This means that it is a group 2 element. Barium's atomic number is 56; this means that it has 56 protons in its nucleus and also.




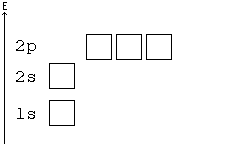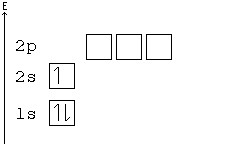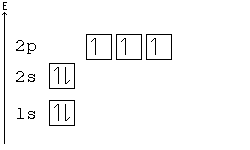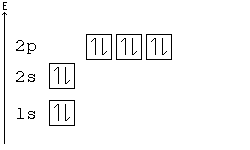

















0 Response to "40 orbital filling diagram for calcium"
Post a Comment