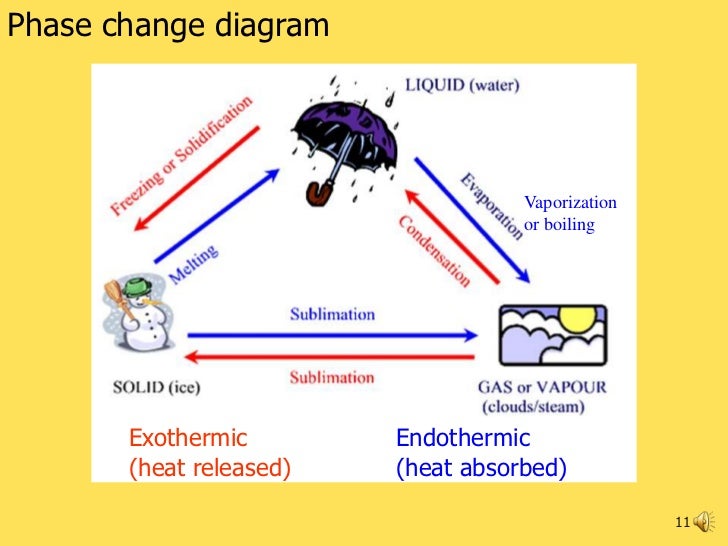
41 phase change diagram endothermic exothermic
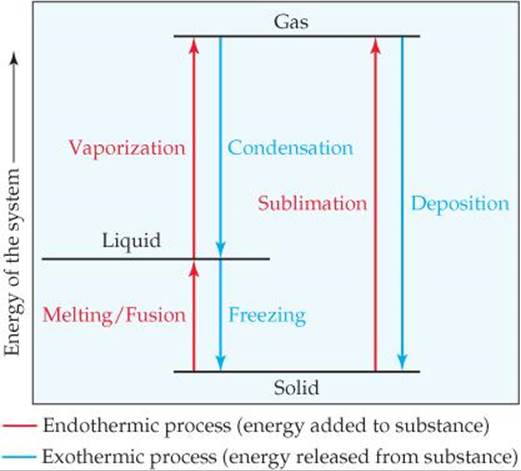
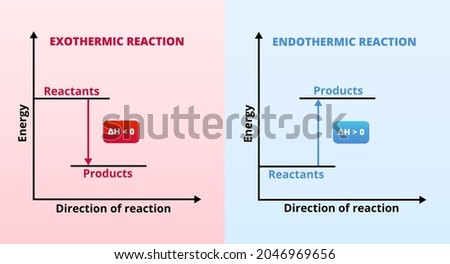
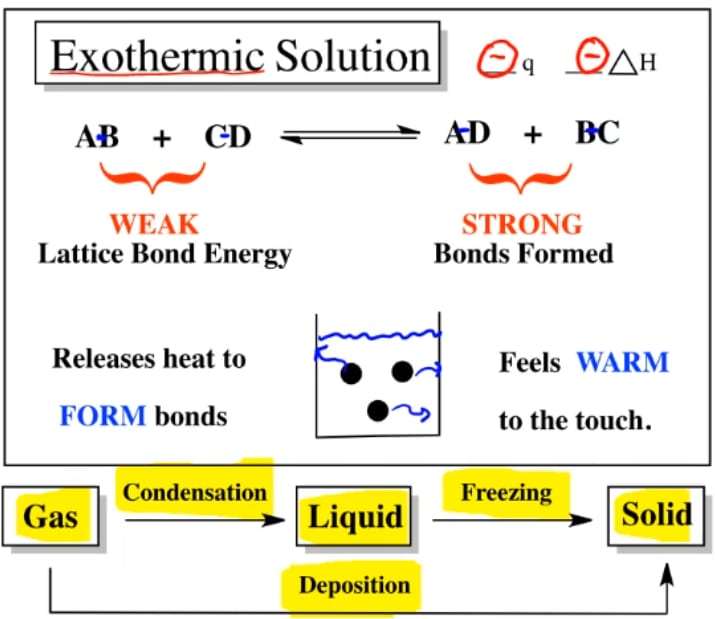
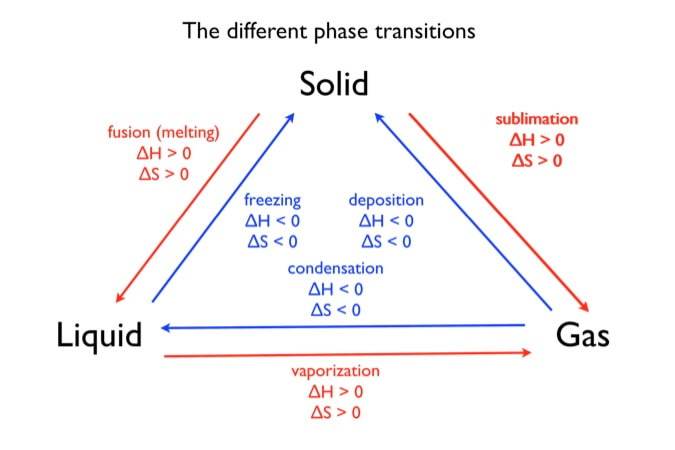
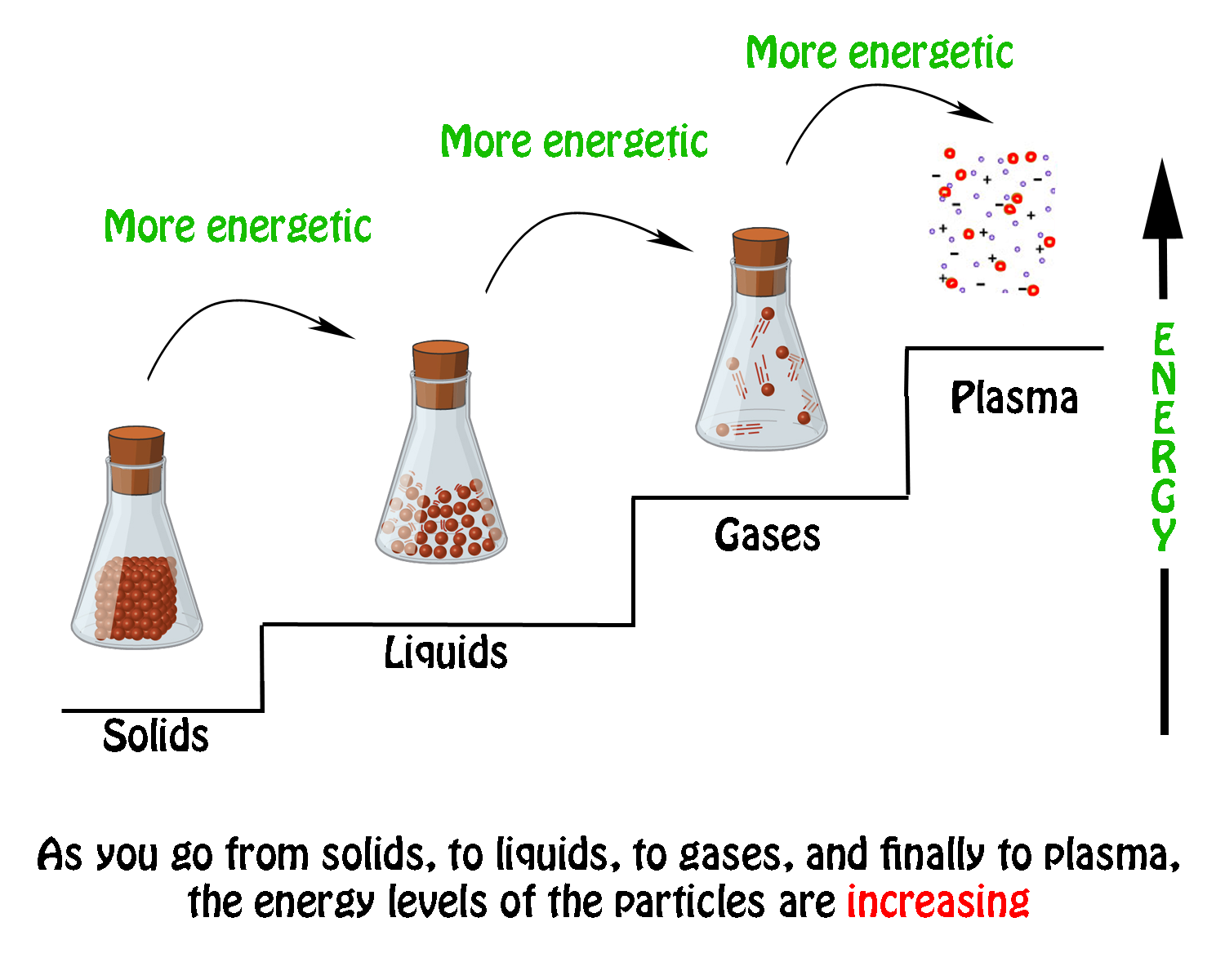
Recall that endothermic processes have a positive enthalpy change, and exothermic processes have a negative enthalpy change. Thermodynamic (Macroscopic) View In addition to the microscopic view presented above, we can describe phase transitions in terms of macroscopic, thermodynamic properties. Endothermic processes require an input of energy to proceed and are signified by a positive change in enthalpy. Exothermic processes release energy upon completion, and are signified by a negative change in enthalpy. Key Terms. exothermic: Of a chemical reaction that releases energy in the form of heat.
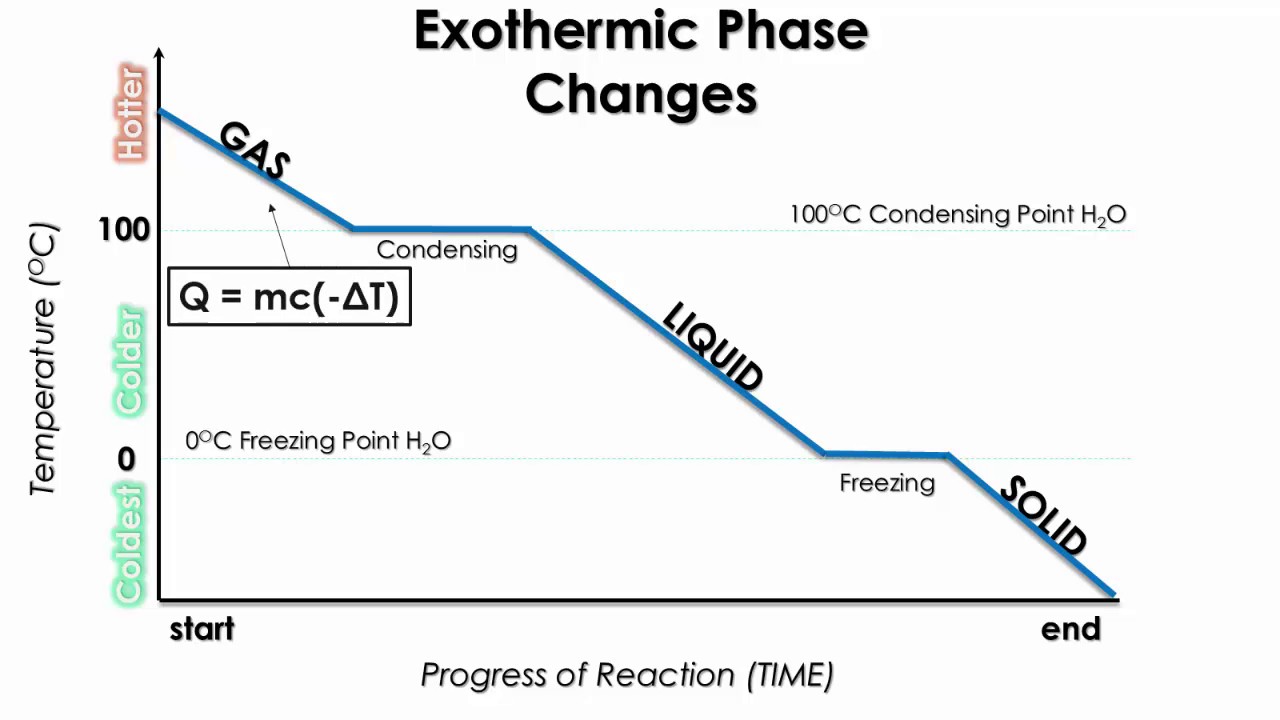
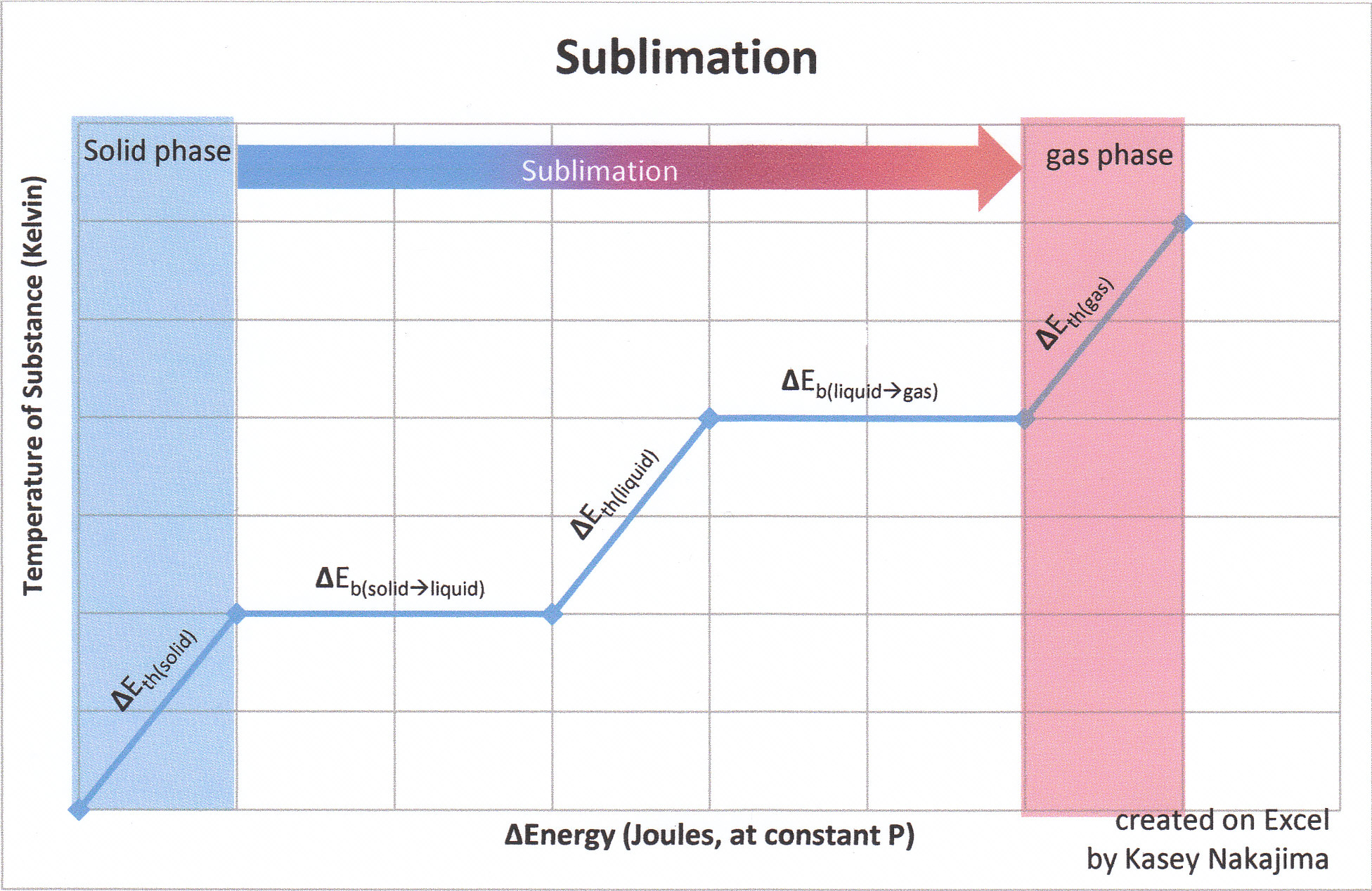
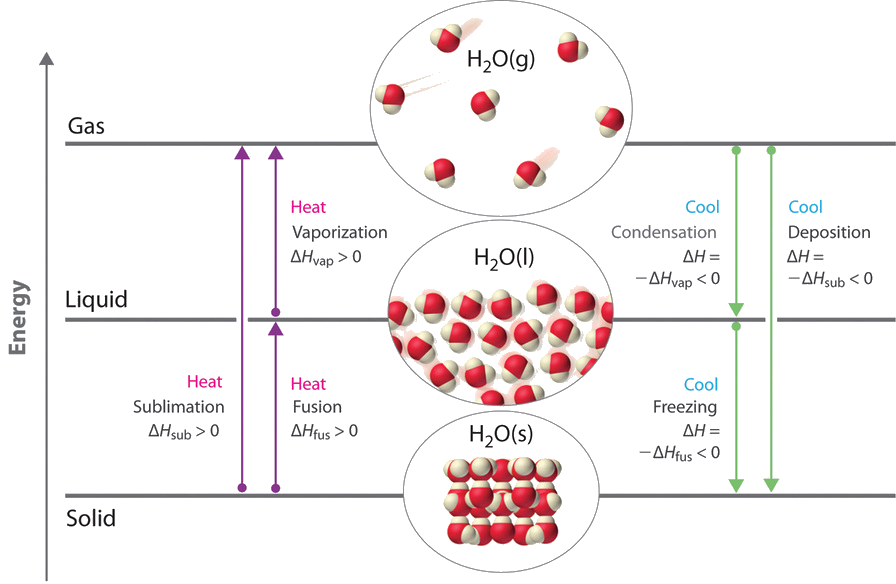
6 Nov 2021 — Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes.Energy Changes That... · Temperature Curves · Heating Curves
Phase change diagram endothermic exothermic
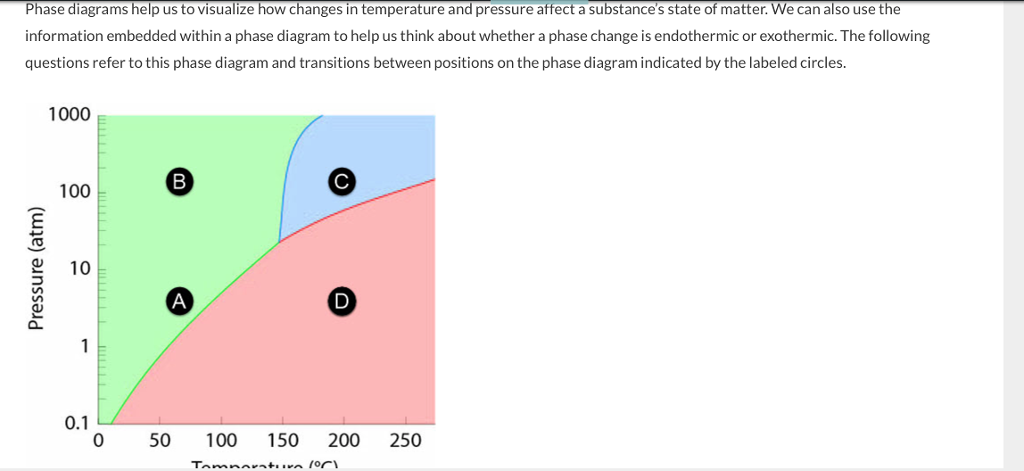
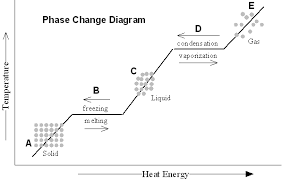
Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer. during phase changes in the diagram below. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the end of your textbook. Endothermic Exothermic Characteristics of Phase Changes (pages 84–86) 1. What is a phase change? 19 Mar 2003 — When attempting to understand the phase changes it is important to remember what is occurring in endo and exothermic reactions. Endothermic - ...
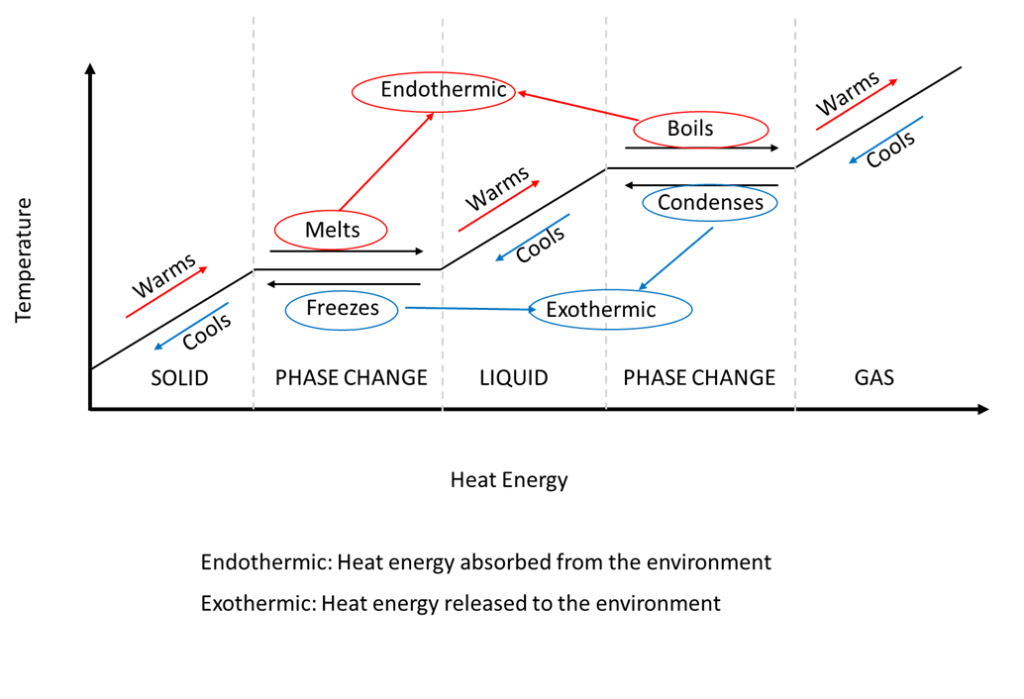
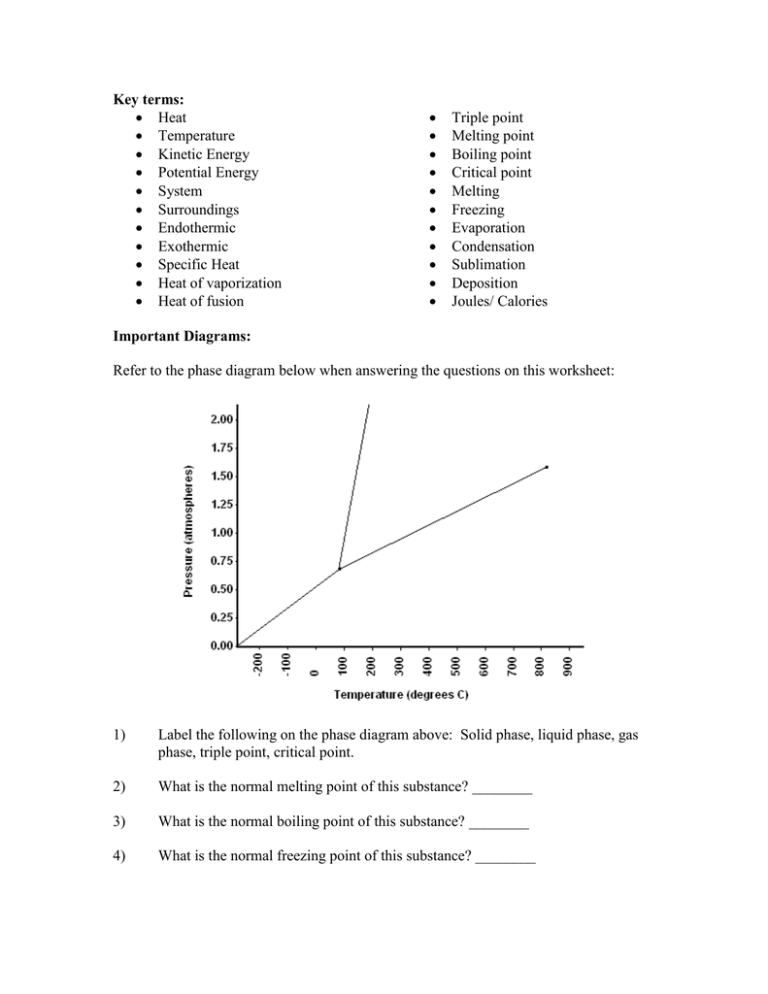
Phase change diagram endothermic exothermic. Label this on the diagram. (40.7 kJ per mol for water) Phase Change Diagram. The graph was drawn from data collected as 1 mole of a substance was heated at a constant rate. Use the graph to answer the following questions. Use the phase change diagram above to answer the following questions. Describe what is occurring from; A to B. B to C. C to ... This diagram shows the names of the phase transitions between solids, ... Recall that endothermic processes have a positive enthalpy change, and exothermic ... 14 Feb 2020 — Here is how you would classify the phase changes as endothermic or exothermic: melting, evaporation and sublimation are endothermic ... Thermochemistry · Practice: Thermochemistry questions · Phase diagrams · Enthalpy · Heat of formation · Hess's law and reaction enthalpy change · Gibbs free energy ...
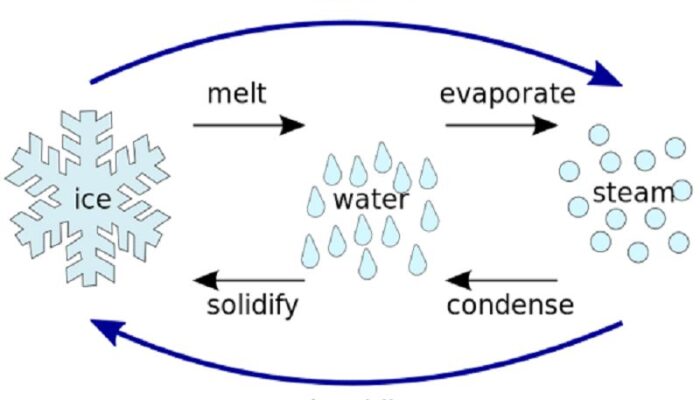
The phase change between a liquid and a gas has some similarities to the phase change between a solid and a liquid. At a certain temperature, the particles in a liquid have enough energy to become a gas. The process of a liquid becoming a gas is called boiling (or vapourization), while the process of a gas becoming a liquid is called ... Substances can change phase—often because of a temperature change. ... Because the substance is melting, the process is endothermic, so the energy change ... Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. 19 Mar 2003 — When attempting to understand the phase changes it is important to remember what is occurring in endo and exothermic reactions. Endothermic - ...
during phase changes in the diagram below. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the end of your textbook. Endothermic Exothermic Characteristics of Phase Changes (pages 84–86) 1. What is a phase change? Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.
















0 Response to "41 phase change diagram endothermic exothermic"
Post a Comment