42 diagram of exothermic reaction
hi guys imma just type the summary of things we need to know for chem o lvl's tmr (pls add on in the comments if i miss out something so i can edit this again) in hoping to help out others while also helping me to revise bcos typing notes is wayy faster than writing :) also this wld not be super precise bcos im just typing in the summary if not i'm literally typing a whole textbook here, but i'll try my best to put in all the infos that is important \*totally not last minute\* **kinetic partic...
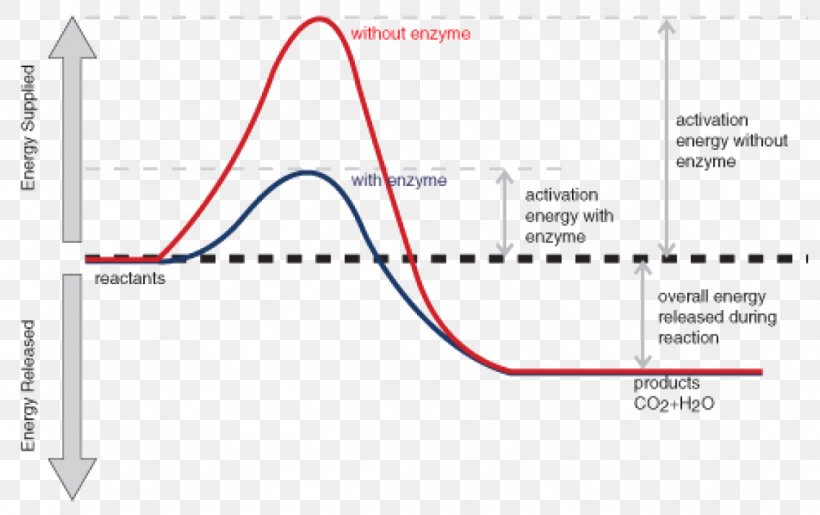
Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
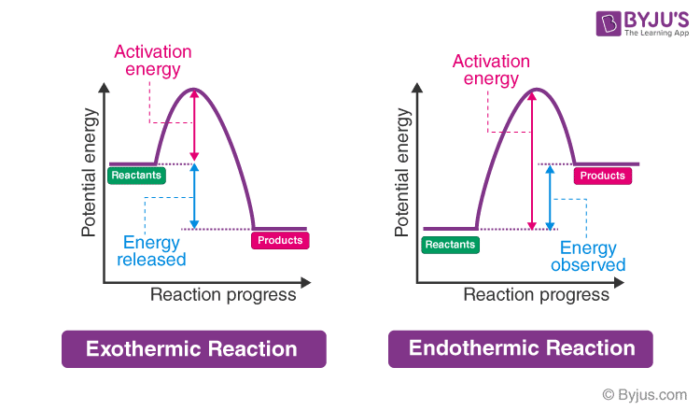
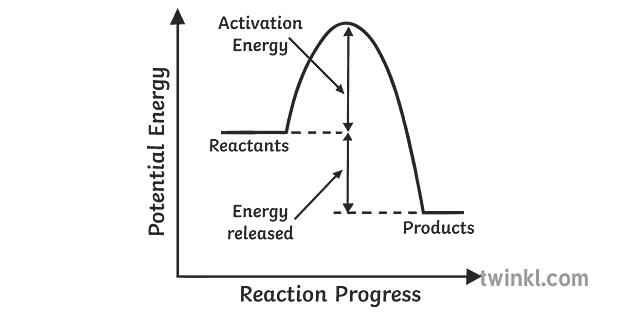
Exothermic Reaction In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products.
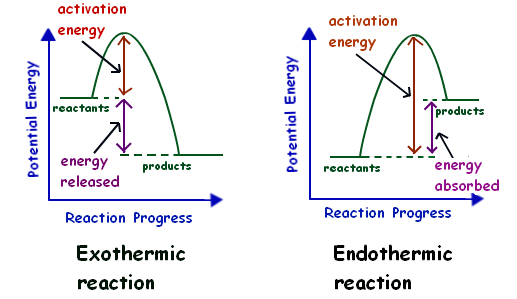
Diagram of exothermic reaction
In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ...
Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Endothermic reactions take in energy and the temperature of the surroundings decreases.
Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction.
Diagram of exothermic reaction.
A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed. Shows whether a reaction is exothermic. Figure shows the energy level diagram for the reaction between methane and oxygen.
The diagram shows a reaction profile for an exothermic reaction. A reaction profile for an exothermic reaction Question. Describe how you can tell from a reaction profile that a reaction is ...
Start studying Chemistry H, Venn Diagram {Exothermic vs. Endothermic} :). Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Hey chemhelp, For chemistry I am doing a Report on Reaction Rates. I am almost complete but need help with the last two things. First - finding the ∆H for the following: 2HCl(aq) + Mg(s) -> MgCl(aq) + H2 = -∆H ? Even if you can just help me with the formula, it was 3 cm of Mg ribbon and 5 differ mol/L of HCl (2.0, 1.5, 1.0, 0.5, 0.0) Secondly - I have created a Energy Diagram (exothermic) I need to explain using tentative language why this is related to the experiment.
​ It is strongly recommended that you start a book "crazy science" to increase your knowledge. There are PDF arc melting in plasma arc cutting plasma cutting with different working gases can cut all kinds of metals difficult to cut by oxygen, especially for colored metals (stainless steel, aluminum, copper, titanium, nickel). Its main advantages are that When cutting the metal with small thickness, the plasma cutting speed is fast, especially when cutting ordi...
MUST KNOW INFORMATION, Test takes place April 28, 7pm pst should be about 30 min long so im Willing to pay 30 bucks ​ Know the signs that a chemical reaction has occurred. Know how to write a balanced chemical reaction. Know how to write a total ionic chemical reaction and a net ionic chemical reaction. Know the strong acids and the strong bases (these break up in aqueous solutions).Know how to use the solubility Rules to determine if salts are soluble or insoluble. Know the di...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
Energy Profile for Exothermic Reactions. The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the ...
Diagram for Endothermic Reaction. Burning methane was an exothermic reaction, now how do we draw the energy diagram for an endothermic reaction? An example of this is when we dissolve ammonium ...
Given the reaction: A + B --> Ca) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for ...
Passage: https://www.dropbox.com/sh/nf334fmscfmp8kr/AAAgRa8RvngKVH4MRiKGtDEGa?dl=0 This diagram is enough IMO: http://puu.sh/oKlTg/c16c6a7602.png Question: http://puu.sh/oKlkg/61c226f25b.png Anwer: http://puu.sh/oKlnt/c1d1d23cce.png First time getting a Le Chatleir's principle question wrong and kinda annoyed. Would love some help on this question! Sorry for all the links - wanted to make sure you guys could read the passage! I sort of understand it, now but I wanted to go th...
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
So I have a process where 10 mol/s of H2 gas and 1 mol/s of benzene gas are fed into a reactor at 350ºC and 5 bar. The products are then fed to a second reactor at 240ºC and 5 bar to yield cyclohexane. The conversion is dominated by the second reactor since the reaction is very exothermic (equilibrium constant for the first reactor is 3 orders of magnitude smaller than the second). My question is, why would you have the first reactor at such a high temperature if the conversion is going to be s...
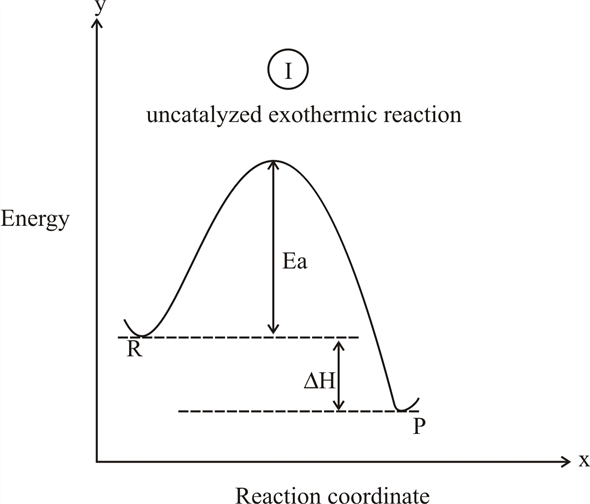
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
10+ Endothermic Energy Diagram. Endothermic reactionin an endothermic reaction, the products are higher in energy than the an energy diagram can be used to show energy movements in these reactions and temperature can be. An energy level diagram shows whether a reaction is exothermic or endothermic. Energy is absorbed δh = + (net gain).
You can start with a generic potential energy diagram for an exothermic reaction. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed. So, the activation energy is the minimum amount of energy required for a reaction to take place. In your case, you need at least "50 ...
Recently a friend of mine (/u/EnthusedDude) purchased two brand new Perlick 650SS flow control taps. Unfortunately, it seems that some [650SS taps are prone to causing a sulfur odour when used with low pH beer or cider](https://www.reddit.com/r/Homebrewing/comments/4411ig/bad_sulfur_smell_caused_by_perlick_flow_control/). In our experience, the sulfur odour happens after the beverage has been sitting in the line for at least an hour; after a pour or two it will go away until it is left stagnant ...
# It's Hive, so I'm sure it's going to be strange. But at its core, their "magic" is still just science[.](https://bungie.net/common/destiny_content/grimoire/hr_images/603040_865612de633ac3e8a3c8c793f6fd0122.jpg) – [Ana Bray](https://www.ishtar-collective.net/transcripts/adventure-deathly-tremors) I’ve wanted to make this post for some time now and have been considering the science of Hive Magic since Stasis was revealed as a Darkness subclass. A while ago I read the post “[Hive soulfire as a p...
Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential energy diagram ...
Headache, woke up later than usual. Only Chem P1 tips today. To those who are currently taking their papers, good luck with them. ​ Second last day, time sure fly fast. Wash up, have a good breakfast, drink some water, whatever it takes to not burn out. Go run an hour before the exam if you wish to, just don't be late. If you forgot your calculator, either borrow from others or learn quick maths. ​ Papers today: \- (1153/1151/1152)/01 Chinese/Malay/Tamil B P1 08:00 - ...
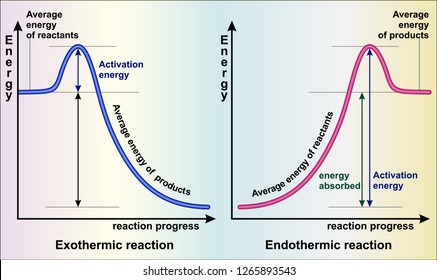
It is helpful to think of exothermic reactions as proceeding from a higher energy (less stable) reactant state to a lower energy (more stable) product state, as shown in the diagram on the right. Reactions in which the products are higher energy than the reactants require an energy input to occur, and are called endothermic. Photosynthesis ...
Jul 9, 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
I don't know if my question is the correct one but a co-worker and I had a debate today. We were working outside and he was complaining that it was very cold. I joked around saying, "I am warm since I have a full belly because I ate too much last night". He explained that having food in your belly doesn't keep you warm or warms you up. He said something along the lines that your body maintains a temp of 98.7 and that you release heat/acquire heat from your extremities; hence, why we put more ...
A guide for alien Species concerning Humans by a Human ​ Written in Union Standard Machine Communication Language (USMCL) by Henry Katou Vizex Machine Translator recommended for reading ​ Section 1: Common misconceptions regarding Hummans ​ I have met individuals of a grand total of 3 non-Human Species and have already discovered a plethora of astonishing misconceptions about my Species. I am a humble molecular network engineer, but I would like to offer t...
Exothermic Diagram. Energy released in bond making. Activation Energy. Energy used in bond. breaking. Exothermic - More energy is released when the products where formed than energy was used to break bonds in the reactants. Therefore, a net release of energy.
Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...
I have a bit of an odd problem: I'm studying how an enzyme catalyzes an oxidation reaction (A + O2 --> B + H2O), so of course I want to make Michaelis-Menten curves, which I usually do by measuring the initial rate of reactant consumption. So, a normal progress of reaction curve should look something like [this:](https://i.imgur.com/ZtB2TAZ.png) But no matter which component I monitor or how, my reactions look like [this](https://i.imgur.com/NBfkPbH.jpg) and [this.](https://i.imgur.com/GmdD...
Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn , as well as DG 1 * and DG 2 * for the first and second activation energies, respectively.
I don't know if my question is the correct one but a co-worker and I had a debate today. We were working outside and he was complaining that it was very cold. I joked around saying, "I am warm since I have a full belly because I ate too much last night". He explained that having food in your belly doesn't keep you warm or warms you up. He said something along the lines that your body maintains a temp of 98.7 and that you release heat/acquire heat from your extremities; hence, why we put more cl...
I've been having trouble with this particular set of questions, I can't tell if I just don't grasp the concepts, or if I actually do know the answers and just can't put it together. 34. Assume that the following reaction is a single step reaction in which a C−Br bond is broken as the C−I bond is formed. The heat of reaction is +38 kJ/mol. I−(aq) + CH3Br(aq) + 38 kJ → CH3I(aq) + Br−(aq)CBrHHHCIHHH a. With reference to collision theory, describe the general process that takes place as this reac...
An exothermic reaction is the release of thermal energy (-ΔH) as it flows out of the system. Thermal energy is negative because energy is being released and the initial potential energy of reactants is more than the energy released from products. Let's explore the exothermic reactions occurring around us: 1.
Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
*Originally posted in* [*/r/DestinyLore*](https://www.reddit.com/r/DestinyLore) # It's Hive, so I'm sure it's going to be strange. But at its core, their "magic" is still just science[.](https://bungie.net/common/destiny_content/grimoire/hr_images/603040_865612de633ac3e8a3c8c793f6fd0122.jpg) – [Ana Bray](https://www.ishtar-collective.net/transcripts/adventure-deathly-tremors) I’ve wanted to make this post for some time now and have been considering the science of Hive Magic since Stasis was r...
Hi all, I was recently listening to the glassware unboxing stream where it was mentioned that /u/ExplosionsAndFire wanted to make xenon trioxide. Before anything, I should mention I am NOT a professional chemist, and I have not attempted any of these reactions myself. I am simply going from my education (minor in chemistry) and the literature. Please ensure you look everything up and take safety precautions if you try this. I propose a slightly round-about method of production: First some war...
Warning: This may be a long post, so bear with me. I currently have some doubts with Kinetics while going over my notes. Consider the following diagram: http://imgur.com/sgCWwFn 1. Why do we have that from the transition state (as it is a "combined" form of the reactants, shouldn't there be "bonds" as well?) we have a potential energy decrease by going to the products? Wasn't it that bond breaking was an endothermic process? If so, why is the case that the potential energy of the system is goi...
Download scientific diagram | Schematic representation of the energy level diagram of an exothermic and endothermic reaction. from publication: Relevance of ...
1. If 6.0 grams of NaCl is dissolved in enough water to make a 300.0-ml solution, calculate the w/v percentage of NaCl. 2. What is the molarity of a solution made from 15.5 grams of NaCl dissolved in enough water to make 1.50 liters of solution? 3. How many grams of HNO3 are there in 75.0 ml of 1.25 M HNO3 solution? 4. What is the concentration of a solution made from 23.5 ml of 8.95 M HCl diluted to 95.0 ml? 5. Which solution will have the lowest freezing point: 1 M NaCl, 1 M M...
I'm tutoring someone who is struggling with topics from content category 5E. I already recommended Leah4Sci and they are already using Jack Westin. What other resources did you like for learning these topics besides simply reading a review book? ​ ***Content Category 5E: Principles of chemical thermodynamics and kinetics*** **Enzymes (BC, BIO)** * [Classification by reaction type](https://jackwestin.com/resources/mcat-content/enzymes/classification-by-reaction-type) * [Mechanism]...
Disclaimer: I'm not interested in debating the science of climate change. If you don't believe in climate change please argue it elsewhere. Thanks! I have an idea for combating climate change, but it involves multiple engineering disciplines which puts most of the feasibility assessment of my idea outside of my expertise. I know myself well enough to know that I'm not cut-out to be an entrepreneur or leader of any sort. My hope is that posting the idea here might inspire some of my fellow engin...
Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between these two energy levels is the energy released to the surroundings, shown as a vertical drop from a higher to a lower level. Because the reactants have more energy than the products they are less stable.
Endothermic vs Exothermic reactions. Exothermic Reaction: When a reaction takes place and it causes the surroundings to heat up or cause the temperature of the surroundings to increase. That reaction is an exothermic reaction. In this type of enthalpy change, the enthalpy of the products is less than the enthalpy of the reactants.
An exothermic reaction is a chemical reaction that releases heat and has a negative enthalpy (-ΔH) and positive entropy (+ΔS).. These reactions are energetically favorable and often occur spontaneously, but sometimes you need a little extra energy to get them started.
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.". Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic.































0 Response to "42 diagram of exothermic reaction"
Post a Comment