38 calculate bond order from mo diagram
Draw the molecular orbital diagram of dioxygen and calculate bond order. Medium. View solution > Explain the molecular orbital structure, bond order, stability and magnetic behavior of Hydrogen molecule on the basis of molecular orbital theory. ... Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic. ... 3.6k views. asked Dec 17, 2020 in Chemical Bonding by Panna01 (47.3k points) closed Dec 18, 2020 by Panna01. Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O 2 is ... It has two unpaired electron in ...
We'll need details about the molecule's molecular orbital diagram to figure out the bond sequence. The electrons are present in bonding orbitals and which are ...

Calculate bond order from mo diagram
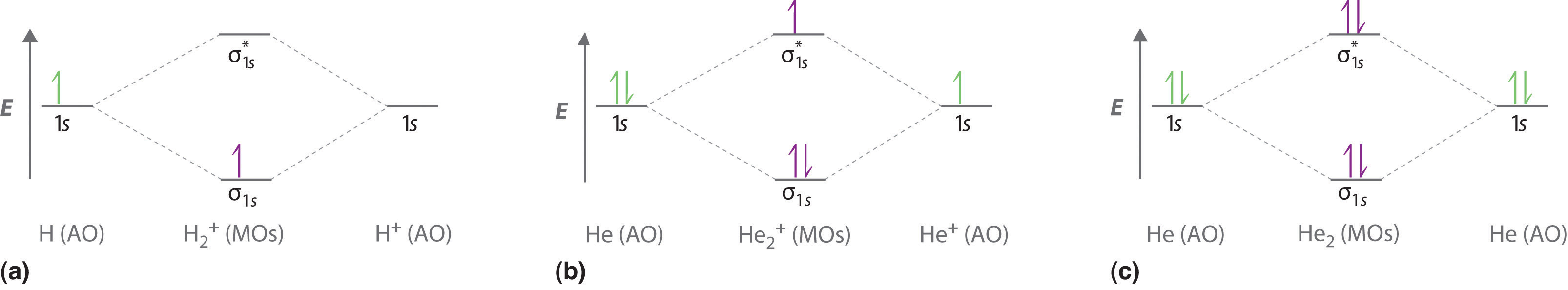
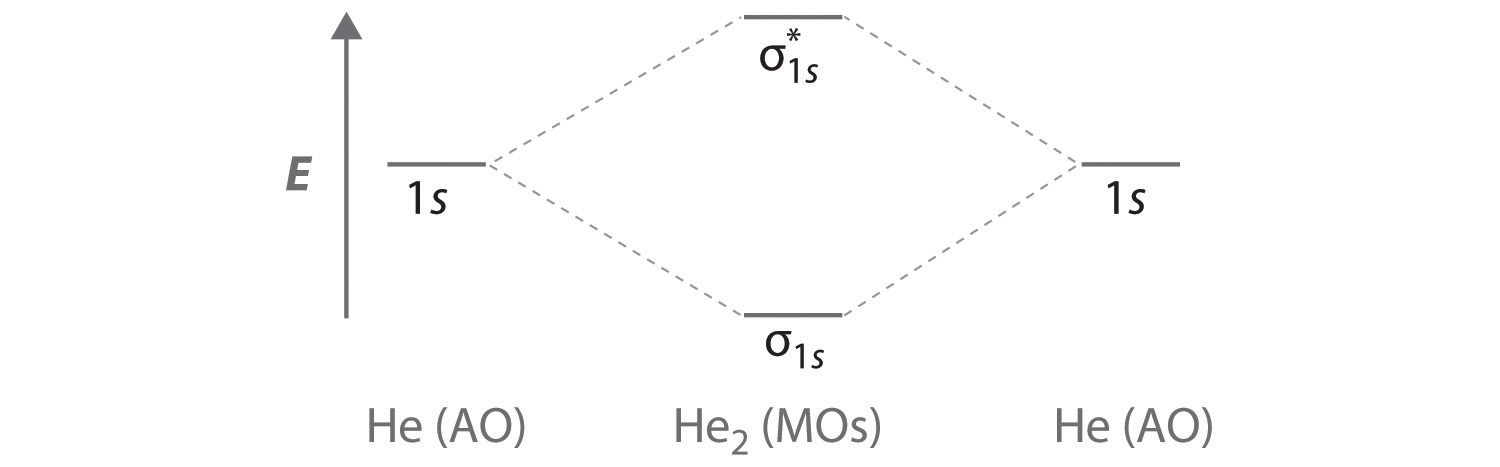
Bond order = n bonding electrons − n antibonding electrons 2 Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B. O. = 8 − 2 2 = 3 Here's a guide on how to construct MO diagrams, in case you need help. 57.6K views View upvotes View 1 share Kakali Ghosh , High School Teacher at Schools The bonding diagram for the hypothetical molecule He2.: Notice the two electrons occupying the antibonding orbital, which explains why the He2 molecule does not exist. ... Bond order is the number of chemical bonds between a pair of atoms. ... Calculate a molecule’s bond order given its molecular ... Draw MO diagram of CO and calculate its bond order.
Calculate bond order from mo diagram. How do I calculate the bond order of HF from a molecular orbital diagram? in HF total no .of electrons are 1+9=10 so, by MOT, sigma 1s2 sigma star 1s2 sigma 2s2 sigma star 2s2 pi 2px1=pi2py1 therefore, bond order= BMO-ABMO/2=6-4/2=1 (ANS) 173 views Answer requested by Jean Joandy Perrine Related Answer Vivek Kejriwal Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Your …. View the full answer. Transcribed image text: Question 20 of 46 Use the MO diagram (below) to calculate the bond order for Iz. Previous question Next question. March 13, 2019 - write the molecular orbital diagram of n2 and calculate their bond order - Chemistry - TopperLearning.com | qbqjy Solved Example Solution Step 1. Write the electron configuration of C2 molecule. Electronic configuration of C2 = (σ2s) 2 (σ * 2s) 2 n (2px) 2 n (2py) 2 Step 2. From the above electron configuration, but the values in the formula Bond order = (Nb - Na) / 2 = ( 8 - 4 )/ 2 = 2
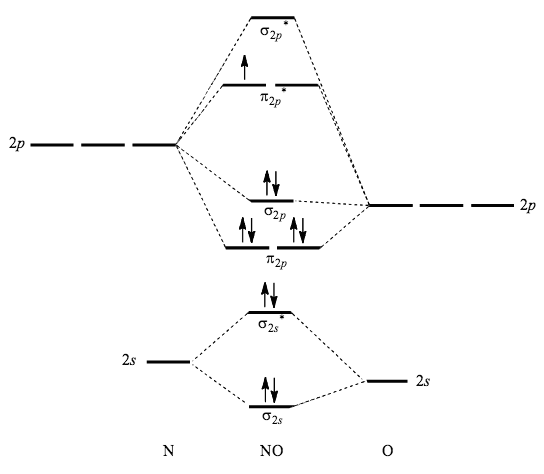
Using the MO diagram of calculate the bond order. Compare it to Answer: The MO diagram for is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being -like, see here and comments.) Quick overview of what […] What you need to understand are the periodic trends and how to affect bond lengths. When calculating bond orders, you use the equation Bond order= [ (# of bonding electrons)- (# of antibonding electrons)]/2. The way you find the number of bonding and antibonding electrons is by understanding and using the appropriate molecular orbital diagram. Use the MO diagram (below) to calculate the bond order for BO: Satyam G. Jai Hind College. Answer. Use the MO diagram (below) to calculate the bond order for BO: Discussion. You must be signed in to discuss. Video Transcript. we are having a model we are having We are having a baron. Sorry for this Baron Baron 1- 2 to be one. From complete energy - level diagram, we can calculate bond order, defined as one - half net number of bonding electrons. In bond orders, electrons in antibonding molecular orbitals cancel electrons in bonding molecular orbitals, while electrons in nonbonding orbitals have NO effect and are not count.
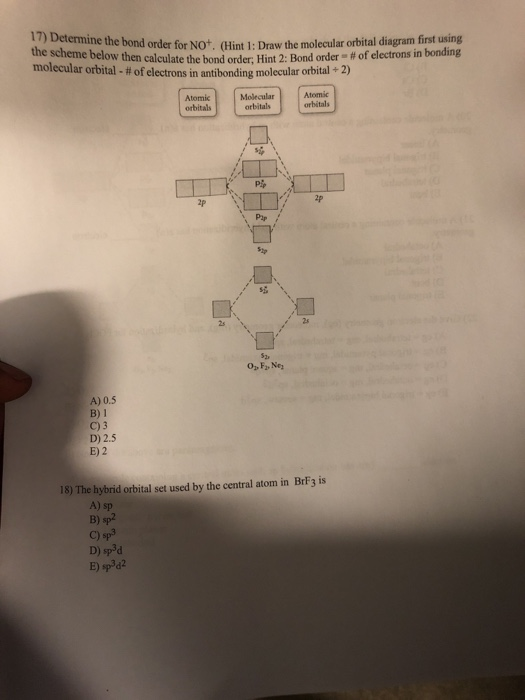
Describe traits of bonding and antibonding molecular orbitals; Calculate bond orders based on molecular electron configurations; Write molecular electron ... Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.com/l.php?u=https://fb.me/ Now, you have to take a deep look at the above molecular orbital diagram of o2 molecule. There is a formula for calculating bond order, is. [ (number of electrons in bonding orbitals ) - (number of electrons in antibonding orbitals)] ———————————————————. 2. O2 Bond Order = [ (8)- (4)]/2 = 4/2 = 2. Due to ... March 18, 2018 - The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being "N"_2-like, see here and comments.) Quick overview of what the labels correspond to what ...
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in .
Draw MO diagram of CO and calculate its bond order .
Electronic configuration of CO molecule is: σ1s2 σ*1s2 σ2s2 σ*2s2 π2py2 π2pz2 π2px2 3. Bond order = = N b−N a 2 N b − N a 2 = 10−4 2 10 − 4 2 = 3 4. Molecule has no unpaired electron, hence it is diamagnetic. ← Prev Question Next Question → Find MCQs & Mock Test Free JEE Main Mock Test Free NEET Mock Test Class 12 Chapterwise MCQ Test
Finding Bond Order Quickly 1 Know the formula. In molecular orbital theory, bond order is defined as half of the difference between the number of bonding and antibonding electrons. Bond order = [ (Number of electrons in bonding molecules) - (Number of electrons in antibonding molecules)]/2. 2
📗 Need help with chemistry? Download 12 Secrets to Acing Chemistry at http://conquerchemistry.com/chem-secrets/💯 If you like my teaching style and are inte...
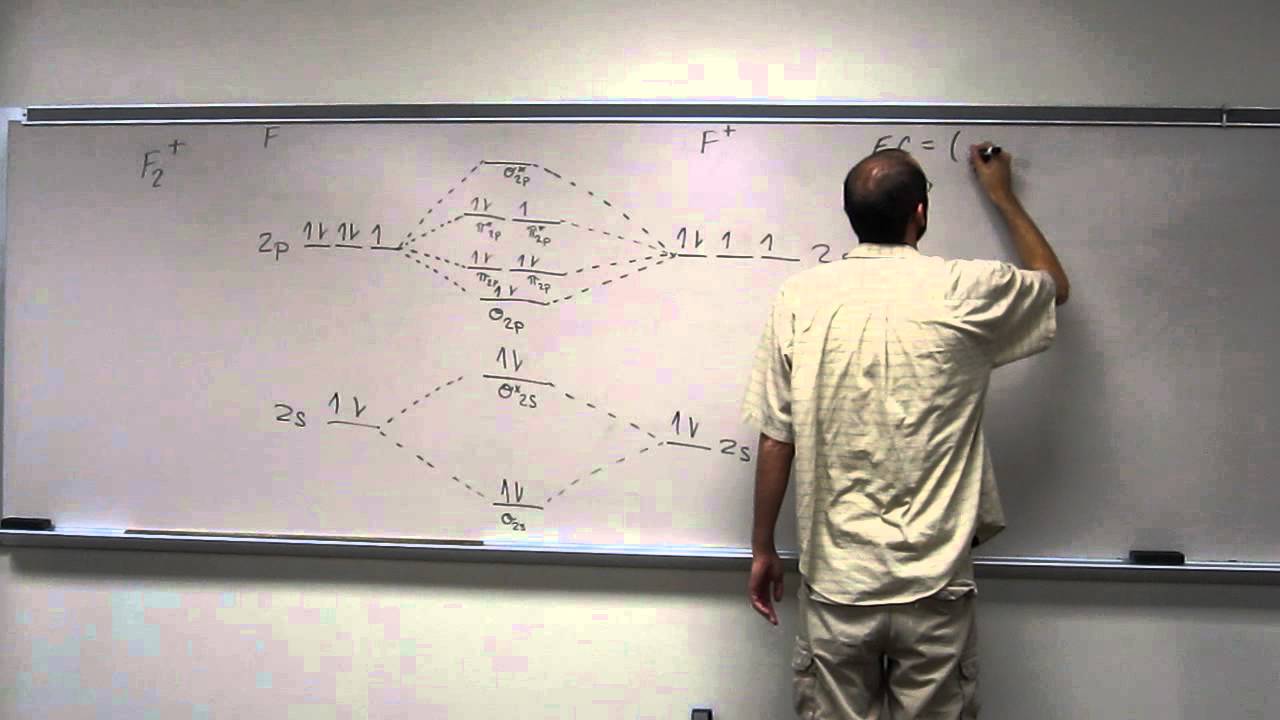
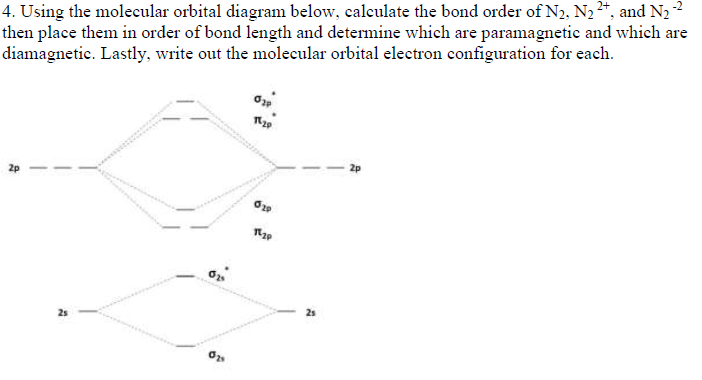
Draw the molecular orbital diagram of N2 and calculate the bond order.
Bond order = Number of bonding electrons − Number of antibonding electrons 2 then, yes, it won't be able to capture the subtleties that you describe. After all, when you use that, you're categorising electrons into bonding vs antibonding in a black and white manner. So it's really no surprise that it doesn't reflect "weakly bonding" MOs.
The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]` The two formulas for bond order tell us the same information.
Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare...
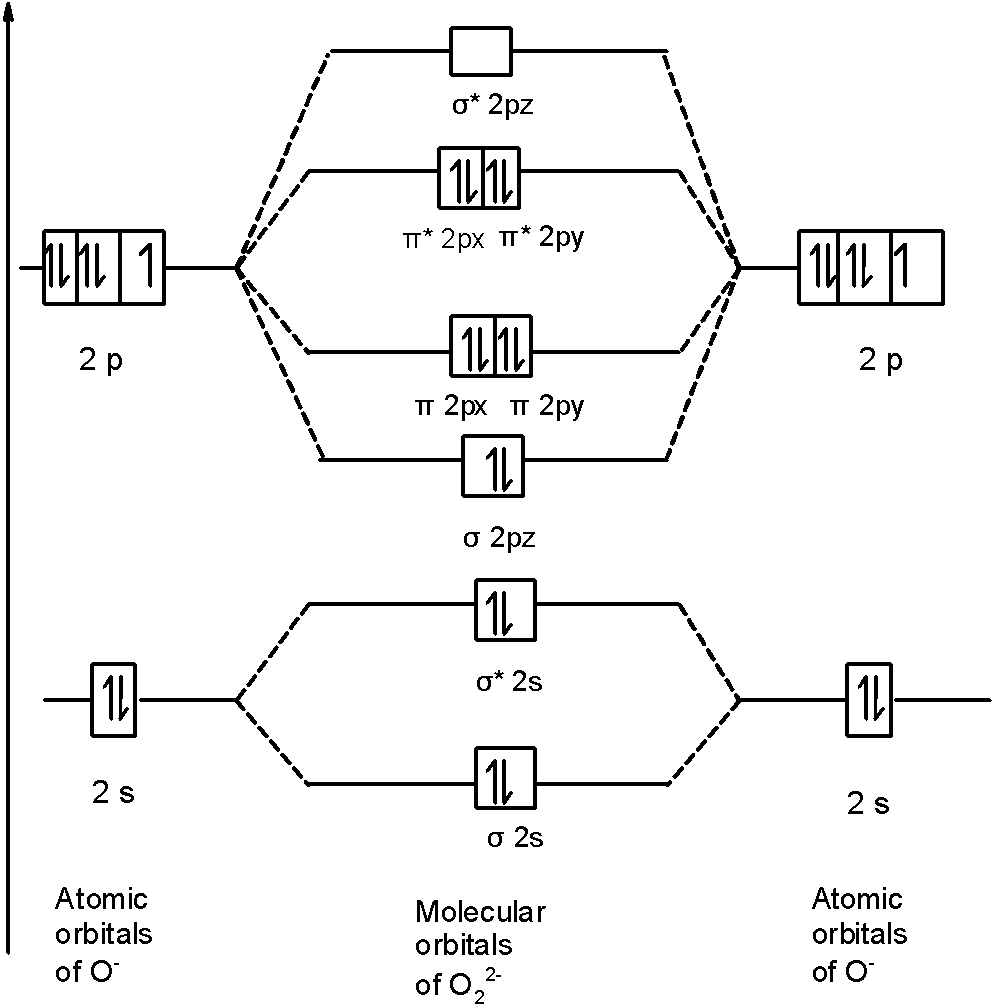
It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.Check me out: http://www.chemistnate.com
site map
In molecular orbital theory, we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital cancel the effect of one bond. We can calculate the bond order in the O2molecule by noting that there
Electron Configurations and Bond Orders Just as with atoms, we can write a molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O–O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons.
Does it start with looking where the double bond is and the group on the double ... A: The IUPAC name of the given compuond is given below: ... A: Entropy measure randomness or disorderness of molecules Randomness increase, entropy also increased ... Q: Calculate MW from Average EM.
Click here👆to get an answer to your question ✍️ Using the MO diagram of NO, calculate the bond order. Compare it to NO^ + ?
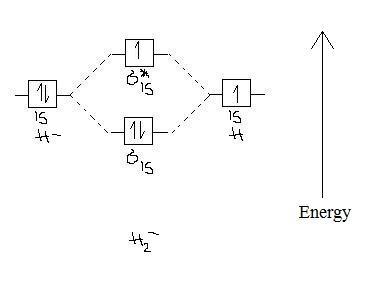
Calculate the Bond Order applying the formula in cell J4: = (H4 - I4) / 2 Example 3 Determine Bond order for hydrogen gas (H 2 ). Also, draw its molecular Orbital Diagram by using Molecular Orbital theory. No of Bonding electron = C4+D4
write the molecular orbital diagram of n2 and calculate their bond order - Chemistry - TopperLearning.com | qbqjy Practice Test - MCQs test series for Term 2 Exams ENROLL NOW
In this example problem, we show how to fill a molecular orbital diagram for a diatomic molecule and use molecular bond theory to compare bond order, bond st...
The bond order is calculated by subtracting the destabilizing (antibonding) electrons from the stabilizing (bonding) electrons. Since a bond consists of two electrons, we divide by two to get the bond order. We can determine bond order with the following equation:
To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
A molecular orbital (MO) diagram shows all the MO orbitals that were formed from the combination of atomic orbitals. MOs that are lower in energy strengthen ...
Click here👆to get an answer to your question ️ Draw the molecular orbital diagram of dioxygen and calculate bond order. Solve Study Textbooks. Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure ... In the molecular orbital diagram for the molecular ion, N 2 + ...
How do I calculate the bond order of HF from a molecular orbital diagram? in HF total no .of electrons are 1+9=10 so, by MOT, sigma 1s2 sigma star 1s2 sigma 2s2 sigma star 2s2 pi 2px1=pi2py1 therefore, bond order= BMO-ABMO/2=6–4/2=1 (ANS) 173 views Answer requested by Jean Joandy Perrine Related Answer Vivek Kejriwal
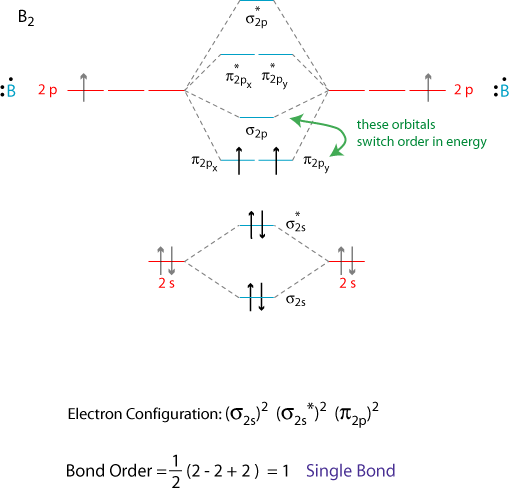
Bond order = 1 2 ( Number of electrons in BMO) − ( Number of electrons in ABMO) From the diagram, we can see that the number of electrons in bonding molecular orbitals are 4 and the number of electrons in antibonding molecular orbitals are 2. Thus, Bond order = 1 2 ( 4 − 2) Bond order = 1 Thus, the bond order for B 2 molecule is 1.
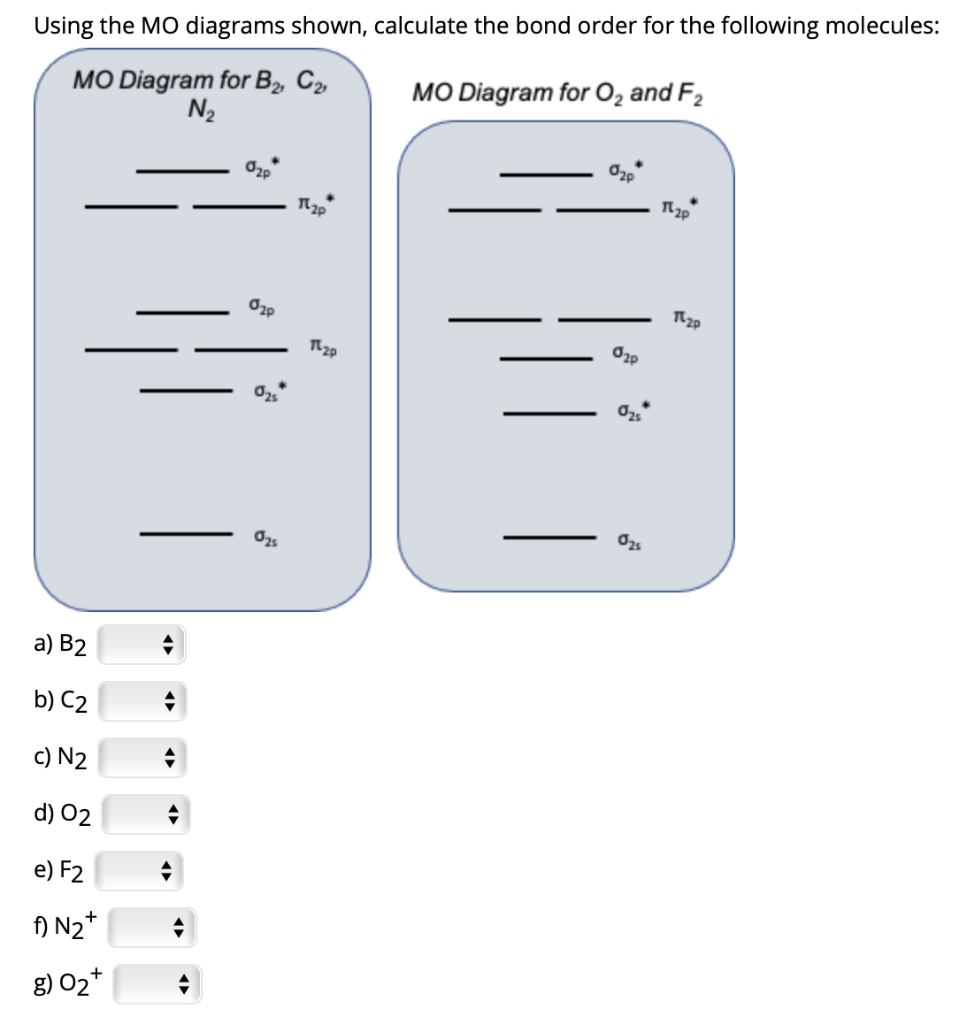
Indicate bond order in the box below for each and circle the correct magnetism. Question: Using the MO diagrams below to calculate bond order and magnetism (dia or para) for each of the following 2 ions? Indicate bond order in the box below for each and circle the correct magnetism.
January 20, 2020 - In this bond order calculator we will show you how to find the bond order using Lewis structures, or a formula derived from the molecular orbital theory.
So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond.
Draw MO diagram of CO and calculate its bond order.
The bonding diagram for the hypothetical molecule He2.: Notice the two electrons occupying the antibonding orbital, which explains why the He2 molecule does not exist. ... Bond order is the number of chemical bonds between a pair of atoms. ... Calculate a molecule’s bond order given its molecular ...
Bond order = n bonding electrons − n antibonding electrons 2 Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B. O. = 8 − 2 2 = 3 Here's a guide on how to construct MO diagrams, in case you need help. 57.6K views View upvotes View 1 share Kakali Ghosh , High School Teacher at Schools

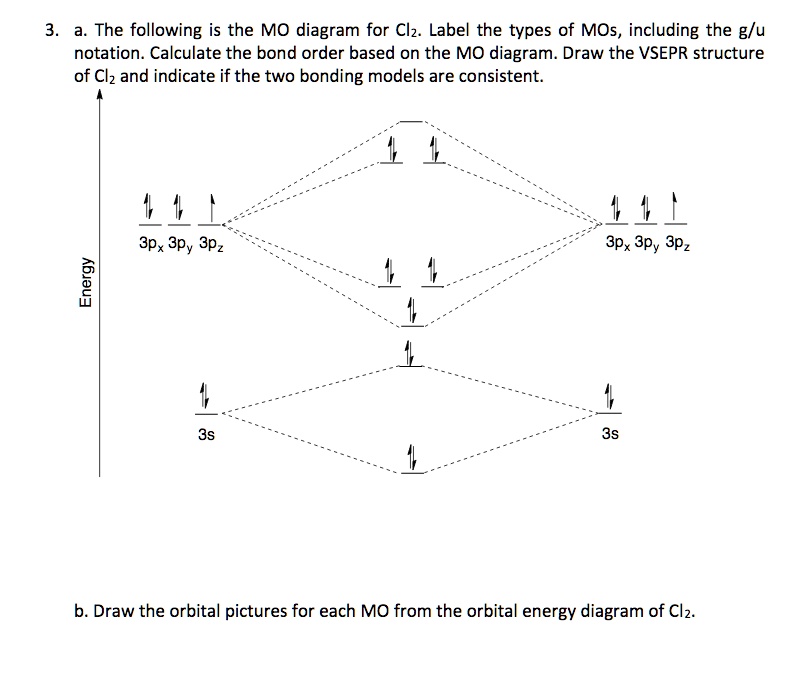





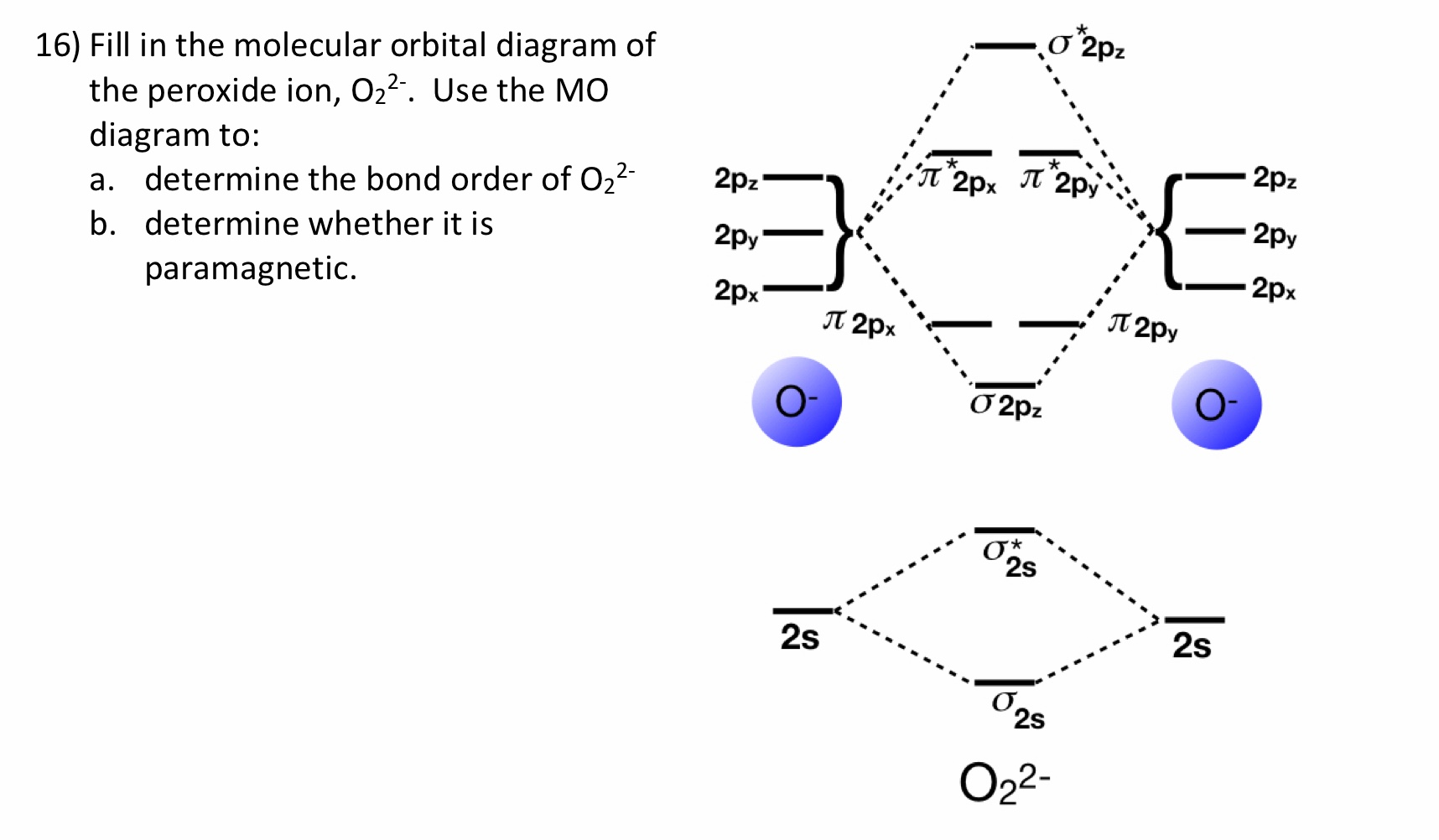
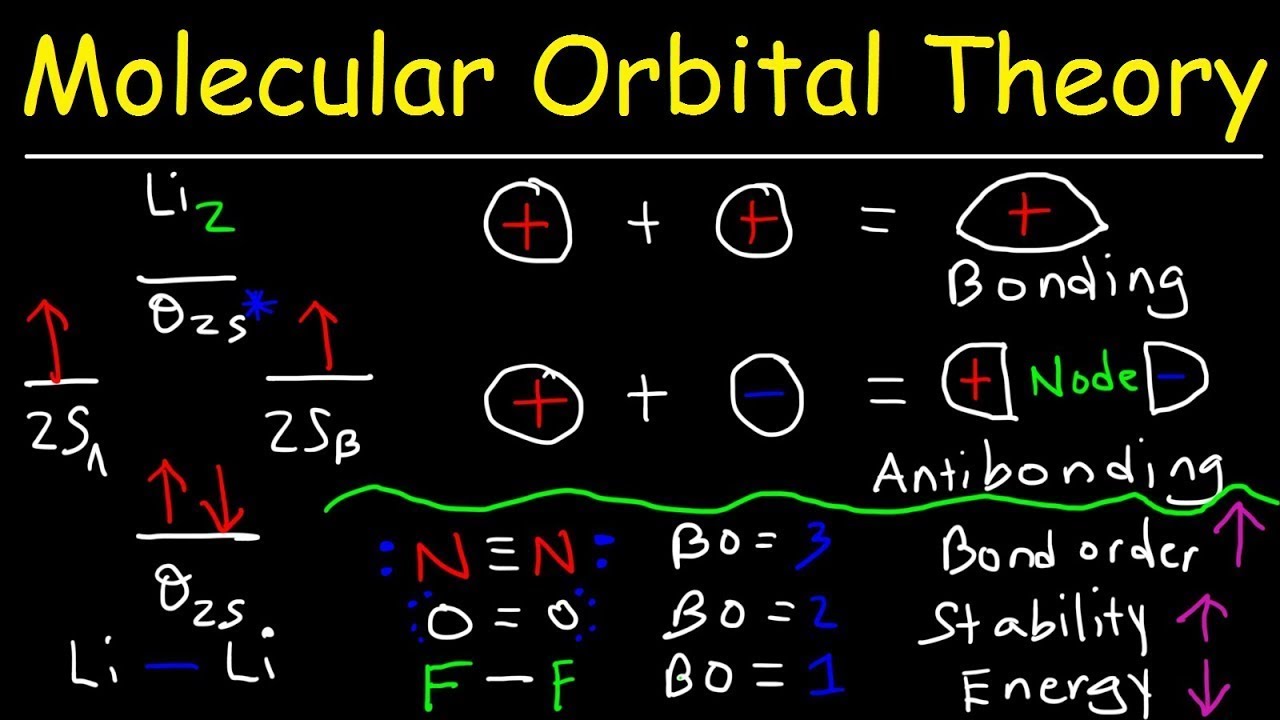
![Best Answer] draw the molecular orbital diagram of N2 and ...](https://hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)
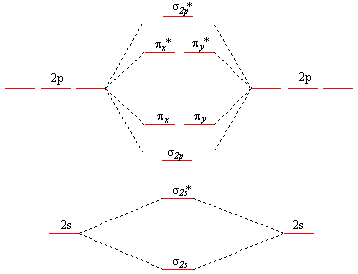












0 Response to "38 calculate bond order from mo diagram"
Post a Comment