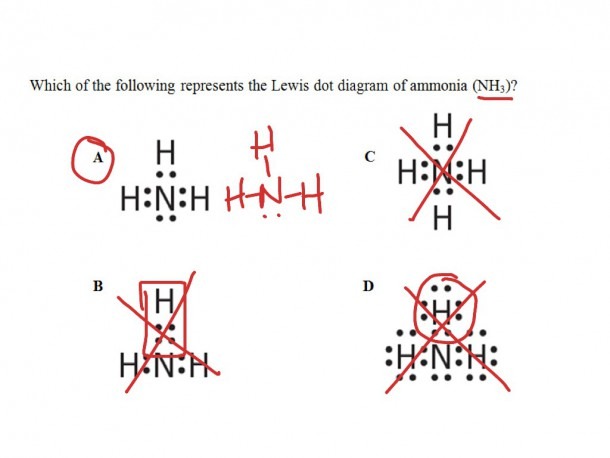
40 electron dot diagram for nh3
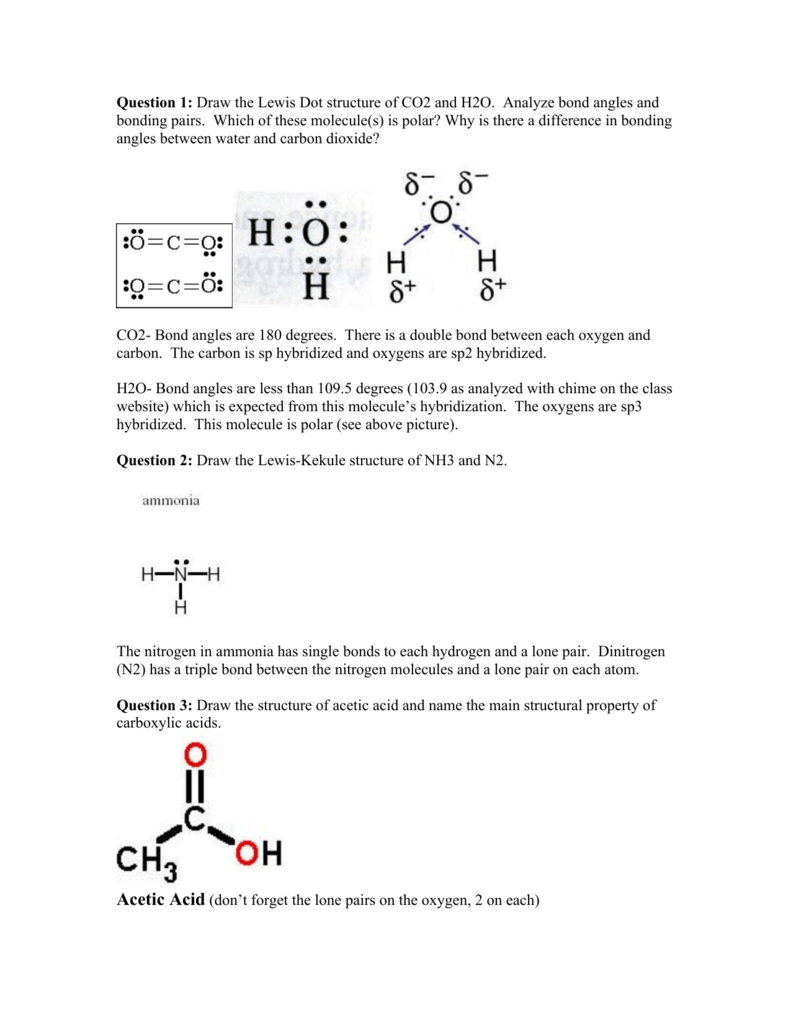
Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Steps of drawing the lewis structure of NH3 is explained in detail in this tutorial. The Lewis structure of ammonia, (NH3) would be three hydrogen atoms which are bonded to a nitrogen atom in the middle with one lone pair of electrons on top of ...
Draw an electron dot diagram to show the formation of ammonium ionAtomic number N7 and H1
Electron dot diagram for nh3
The structure looks like this: · First Nitrogen tends to form 3 covalent bond with 3 Hydrogen atoms which produces NH3. · After the formation of NH3 there is ...3 answers · 2 votes: Electrons of N is shared by H to complete its duplet and elctrons of H is shared by N to ... Electron Dot Notation - 9 images - lewis dot structure for boron atom b youtube, silicon electron configuration youtube, ... We give a positive response this nice of Electron Dot Notation graphic could possibly be the most trending subject behind we allowance it in google gain or facebook. In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
Electron dot diagram for nh3. Ammonia (NH3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a ...25 Oct 2016 · Uploaded by Wayne Breslyn May 5, 2018 - Have a look here... The Lewis structure of ammonia, NH_3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. We have previously shared a detailed blog on the NH3 Lewis structure that you can check out for a quick revision of its lewis dot structure. Ammonia has three single covalent bonds formed between the Nitrogen atom and the Hydrogen atoms, along with one pair of nonbonding electrons on the nitrogen atom. \The electron dot diagram for a neutral atom of chlorine (atomic number 17) is shown below. Which of the following symbols represents a chlorine ion with a stable arrangement of eight valence electrons? A. 35Cl1-B. 35Cl2-C. 35Cl1+ D. 35Cl
NH3 + H+ ——> NH4+ Lewis Structure. Lewis Structure is a simplified arrangement and presentation of the electrons present in the valence shell of a molecule. A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. In the Lewis Structure, electrons are depicted as dots. Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. There are 8 valence electrons available for the Lewis structure for NH 3. This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia. My Website: https://www.video-tutor.net Patreon: https:/... In this video I draw the Dot and Cross Diagram for NH3 (Ammonia). Dot-and-cross diagrams are one way to represent covalent bonds in molecules. They are simil...
ShowMe is an open learning community featuring interactive lessons on a variety of topics. hi guys imma just type the summary of things we need to know for chem o lvl's tmr (pls add on in the comments if i miss out something so i can edit this again) in hoping to help out others while also helping me to revise bcos typing notes is wayy faster than writing :) also this wld not be super precise bcos im just typing in the summary if not i'm literally typing a whole textbook here, but i'll try my best to put in all the infos that is important \*totally not last minute\* **kinetic partic... Two students made the lewis dot diagrams of nh3. The diagrams are as shown. Two students made the lewis dot diagrams of nh3. The diagrams are as shown. Categories Uncategorized. Leave a Reply Cancel reply. Your email address will not be published. Required fields are marked * Comment. Valence Shell Electron Pair Repulsion Concept. → Predicting the shape/structure of molecules. It was established by Gillespie and Nyholm in the year 1957.
# Why is A Level Chemistry So Hard? If you are taking A Level Chemistry, you will probably agree, like most students, that A Level H2 Chemistry is difficult and you have good reasons to do so. The concepts are complex and involve much memory work. There is a steep increase in the learning curve. This is only natural. Having now graduated from the top 20% of the O Level cohort, the syllabus is now made much tougher to further differentiate among all of you. # Much Memory Work is Required C...
The electron-dot structure of NH3 is: In NH3, each hydrogen atom shares its one electron with the nitrogen atom to form a covalent bond.
The electron dot structure of the NH3 molecule is also known as the NH3 Lewis ...
Assertion: Ammonia shows a trigonal pyramidal molecular structure. Reason: In the structure of ammonia, three atoms are attached to the central atom and thus, ...
March 1, 2016 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!
January 1, 2019 - The Lewis Dot Structure for NH3 (Ammonia) is shown above. You could also represent the bonds as dots between the two atoms, but this may be confused with the lone pair electrons on the nitrogen.
A step-by-step explanation of how to write the Lewis Dot Structure for NH3 (Ammonia or Nitrogen Trihydride). The Lewis structure for NH3 is one of the most c...
The number of valence-shell bonding electron-dot model for HN3 is: · Number of bonded pairs and lone pairs of electrons present in the central atom of ammonia ...
The lewis structure that is also called an electron dot structure, sa reprsentation de Lewis est la suivante :. Log in with Schéma de lewis nh3. Already have an account. Menu Categories. There are three single bonds schéma de lewis nh3 one lone pair of electrons in NH3 molecule. Ammonia is a colorless compoundused in making fertilizers.
What is the Lewis dot structure for LiH? Is it simply Li - H? Chemistry. Draw a Lewis dot diagram for NF2H and use the oxidation state method of electron bookkeeping to determine how many electrons each atom should be assigned. Chemistry 121. What is the lewis dot structure of C2H5F? chemistry. Hypochlorous acid, HOCl, is a weak acid with Ka ...
Lewis structures are also known as Lewis dot diagrams, Lewis dot structures, Lewis dot formulas, Lewis electron dot structures, or electron dot structures. Basically, these structures or diagrams can be drawn for coordinating compounds and also for a covalently-bonded molecule. ... nh3 (Ammonia) is a toxic gas that can be very dangerous ...
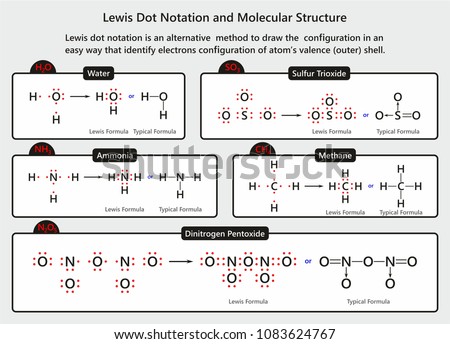
In the space provided below, draw electron-dot diagrams for the following molecules: hydrogen (h2), ammonia (nh3), and methane (ch4). remember that the dots represent the valence electrons. make sure that each atom in the molecules have 8 valence electrons, except hydrogen, which has only 2.
Draw the electron-dot structures of the following compounds and state the type of bonding in each case: (i) KCl. (ii) NH 3. (iii) CaO. (iv) N 2. (v) CaCl 2. Class 10th. Chemistry. Lakhmir Singh and Manjit Kaur.
By looking at the Lewis dot structure of ammonia NH3 molecule the shape of the ammonia molecule is described by which one of the following aTrigonal pyramidal bTshaped cSquare planar dTrigonal planar
The Cl-N-Cl bond angle is ~107.5, All electrons shown on the left dot and VSEPR correctly predicts their V or bent shape and a the Lewis dot and cross electronic diagram used to The electron 'groupings' repel to minimise the potential The dot and cross diagram shows this is another example of three groups of bonding carbon atom, one of its four ...
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape. Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton.
The Lewis structure of the tetra atomic ammonia (NH3) molecule has three single sigma bonds between the nitrogen and the hydrogen atoms. Moreover, the presence of a single lone pair of electrons on the nitrogen atom is responsible for the bent geometrical structure of the NH3 molecule.
Electron Dot Structure of NH3 by Jeff Bradbury - February 17, 2012 - Lewis Electron Dot Structure for ammonia molecule NH3
January 10, 2019 - Step 5: The rest are nonbonding pairs. Subtract bonding electrons (step 3) from valence electrons (step 1). ... Use information from step 4 and 5 to draw the NH3 lewis structure. ... Alternatively a dot method can be used to draw the NH3 Lewis structure.
December 10, 2016 - There are 8 valence electrons to distribute........... And 3xxN-H bonds, and one nitrogen-centred lone pair of electrons.
November 14, 2018 - Find an answer to your question draw the electron dot structure of ammonia molecule and show the formation of Ammonium Ion from meet level all the bonds
The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3.
By drawing an electron dot diagram, show the lone pair effect leading to the formation of ammonium ion from ammonia gas and hydrogen ion. Answer. NH3 has one ...
February 7, 2017 - Answer: You look up “electron dot diagram for ammonia” on google images. It really isn’t that hard.
NaCl + NH3 + CO2 + H20 NaHCO3 + NH4CI Which Lewis electron-dot diagram correctly represents the reactant conta… Get the answers you need, now!
How To Draw A Lewis Dot Structure For Nh3 How to Draw a Lewis Structure - ThoughtCo Jan 29, 2020 · A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps ...
November 30, 2012 - In the top illustration a line ... electron dots. This was re-drawn for neatness sake in the bottom drawing. By bonding covalently with another atom, each atom has a full valence shell of electrons and remain electron neutral. ... Ammonia solution used as a cleaning solution is ammonia gas NH3 which is dissolved ...
We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15 so it has 5 valence electrons ...
The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons on top of the atom. Due to the presence of two lone pairs of electrons that repel bond pairs N-H it acquires a bent V-shape molecular shape with a bond angle of 1045.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
Electron Dot Notation - 9 images - lewis dot structure for boron atom b youtube, silicon electron configuration youtube, ... We give a positive response this nice of Electron Dot Notation graphic could possibly be the most trending subject behind we allowance it in google gain or facebook.
The structure looks like this: · First Nitrogen tends to form 3 covalent bond with 3 Hydrogen atoms which produces NH3. · After the formation of NH3 there is ...3 answers · 2 votes: Electrons of N is shared by H to complete its duplet and elctrons of H is shared by N to ...




![[NL_9849] Dot Diagram Of Co2 Free Diagram](https://static-cdn.imageservice.cloud/6824381/lewis-dot-structures-ppt-video-online-download.jpg)
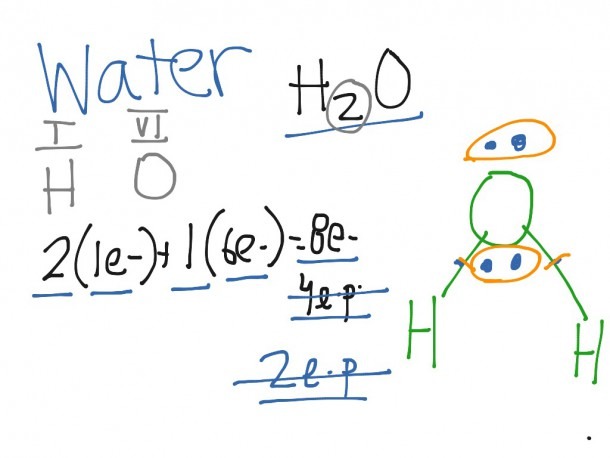







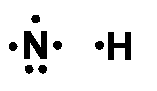




0 Response to "40 electron dot diagram for nh3"
Post a Comment