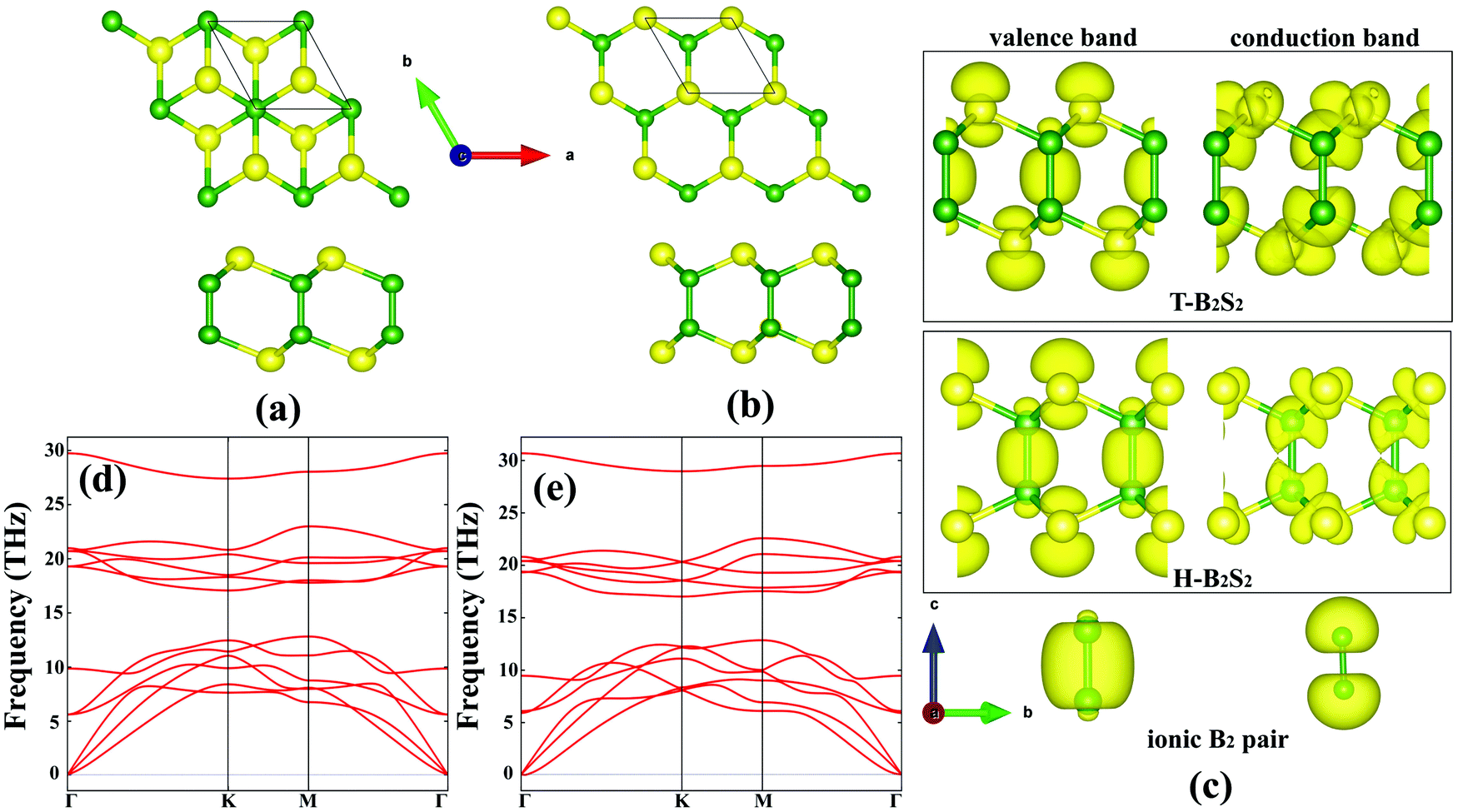
41 molecular orbital diagram b2
(i) Be2 molecule: The electronic configuration of Be(Z = 4) is:4 Be 1s2 2s1Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms.Number of valence electrons in Be atom = 2Thus in the formation of Be2 molecule, two outer electrons of each Be atom i.e. This section contains the course materials for Unit II, including lecture videos, readings, lecture notes, and practice problems.
Multi-resonance thermal activated delayed fluorescence (MR-TADF) has been promising with large oscillator strength and narrow full width at half maxima of luminescence, overcoming the compromise of emission intensity and energy criteria of traditional charge transfer TADF frameworks. However, there are still limited theoretical investigations on the excitation mechanism and systematic ...

Molecular orbital diagram b2
Answer to Molecular Orbital theory predicts that the B2 2- ion would have a bond order of ________. If one of the highest valence ... Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ... Orbital Interaction Theory of Organic ChemistryA Textbook of Inorganic Chemistry - Volume 1Molecular Orbitals and Organic Chemical ReactionsIntroduction to Molecular-orbital TheoryElectronic Absorption Spectra and Geometry of Organic MoleculesA Semi-empirical Mo Theory of Sigma Electron Systems. I. General
Molecular orbital diagram b2. Lecture B2 VSEPR Theory Molecular Orbital Theory. An approach, known as Molecular Orbital Theory, was established primarily by Hund and Mulliken in \(1932\) to explain the features of molecules such as their relative bond strengths, paramagnetic and diamagnetic properties, etc. Molecular Orbital Theory: Introduction, Examples, and More Molecular orbital diagram f2. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. ... B2 2 Molecular Orbital Diagram - Drivenheisenberg. F2 2 ... November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ... cos θ = n, then show that a2 + b2 = m2 + n21 Science N2 And Memos Free PDF ebook Download: Science N2 And Memos Download or Read Online ebook engineering science n2 question papers and memos in PDF Format From The Best ... Draw a molecular orbital diagram of ${N_2}$ or ${O_2}$ with magnetic behavior and bond order. Answer. …1 Science N2 And ...
If a cos θ + b sin θ = m and a sin θ - b cos θ = n, then show that a2 + b2 = m2 + n2 Office Practice N5 Previous Question Papers Draw a molecular orbital diagram of N2 or O2 with magnetic behavior and bond order. Claim your FREE Seat in Vedantu Master Classes! NCERT Solutions for Class 10 Maths. NCERT Solutions for Class 10 Science. Draw a molecular orbital diagram of N2 or O2 with magnetic 15/05/2013 · A Computer Science portal for geeks. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. #1 Free Secondary School Test Papers in Singapore (UPDATED What is the bond order of B2- ? is it paramagnetic or diamagnetic? The blank molecular orbital diagram shown here (Figure 1) applies to the valence of diatomic lithium, beryllium, boron, carbon, or nitrogen. Bonding orbitals are marked with sigma or... Draw a molecular orbital diagram of N2 or O2 with magnetic 1 Science N2 And Memos Free PDF ebook Download: Science N2 And Memos Download or Read Online ebook engineering science n2 question papers and memos in PDF Format From The Best User Guide Database ASSA Maths & Zimsec o level past exam papers pdf Zimsec o level past exam papers with ...
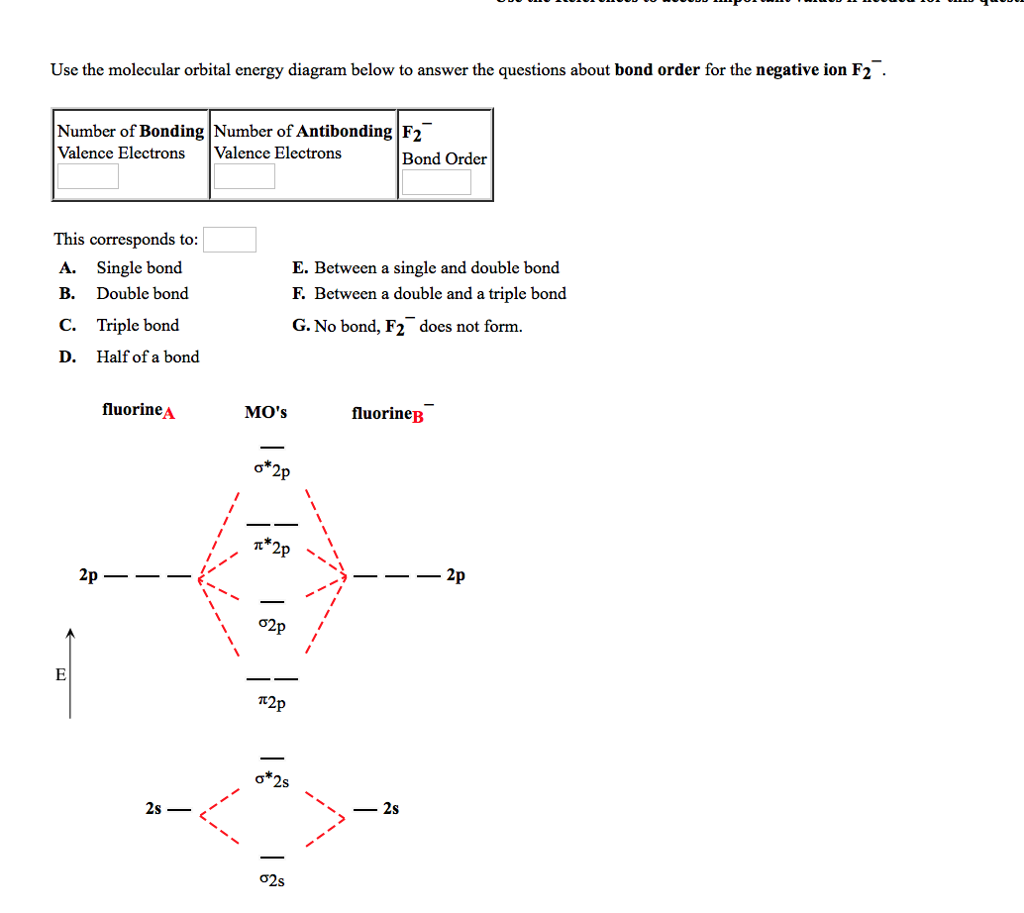
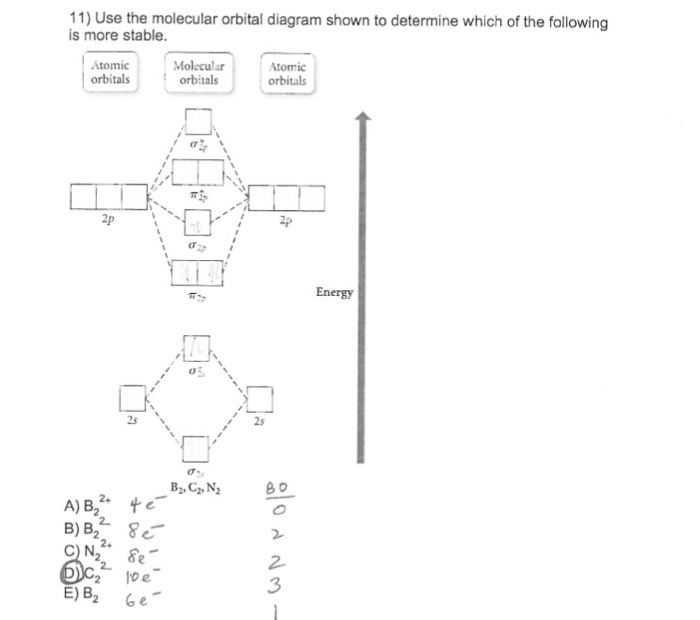
Solution for Below are the molecular orbital diagrams for B2, C2, and N2. Please use it to predict the bond order for B2, B2+ ,B2 -, C2, C2+, C2 -, N2, N2+, and… In B2, C2, and N2, the 𝜎2𝑝 orbital is higher in energy than the 𝜋2𝑝 orbitals as shown in diagram A. In O2 and F2, the 𝜋2𝑝 orbitals are higher in energy than the 𝜎2𝑝 orbital as shown in diagram B. The number of valence electrons per atom of an element is related to the group number in the periodic table. Boron, in group 3A, has 3 valence electrons per atom. Diatomic ... 16/01/2020 · B2 has two unpaired electron so it is paramagnetic whereas C2 has only paired electrons so it is diamagnetic. 28 Related Question Answers Found Is o2 negative paramagnetic? The correct explanation comes from Molecular Orbital theory. The atomic orbitals of the O atoms overlap to form the σ and π orbitals of the O2 molecule as shown in the … The word comet derives from the Old English cometa from the Latin comēta or comētēs.That, in turn, is a romanization of the Greek κομήτης 'wearing long hair', and the Oxford English Dictionary notes that the term (ἀστὴρ) κομήτης already meant 'long-haired star, comet' in Greek. Κομήτης was derived from κομᾶν (koman) 'to wear the hair long', which was itself ...
Use MO diagrams to place B2+,B2, and B2- in order of(a) decr... ... We have solutions for your book! ... JavaScript is required to view textbook solutions. ... Molecular orbitals: Molecular orbitals are formed by linear combination of atomic orbitals. Atomic orbitals and molecular orbitals ...
The Orion Molecular Cloud Complex, or simply the Orion Complex, is comprised of a large group of dark clouds, bright emission and reflection nebulae, dark nebulae, H II regions (large clouds showing recent star forming activity) and young stars in the constellation Orion. The Orion Complex is between 1,500 and 1,600 light years distant. Several parts of it – the famous Orion …
Mo Diagram Bf - 9 images - molek l orbital theorie unterschied n2 und o2 chemie, f2 molecular orbital diagram hanenhuusholli,
So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. In what Orbital does fluorine have an unpaired electron? 2p orbitals
B2 Molecular Orbital Diagram. Collected from the entire web and summarized to include only the most important parts of it. Can be used as content for research and analysis. Home Blog Pro Plans Scholar Login. Advanced searches left . 3/3. Search only database of 12 mil and more summaries ...
B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3. In the formation of B2 ...
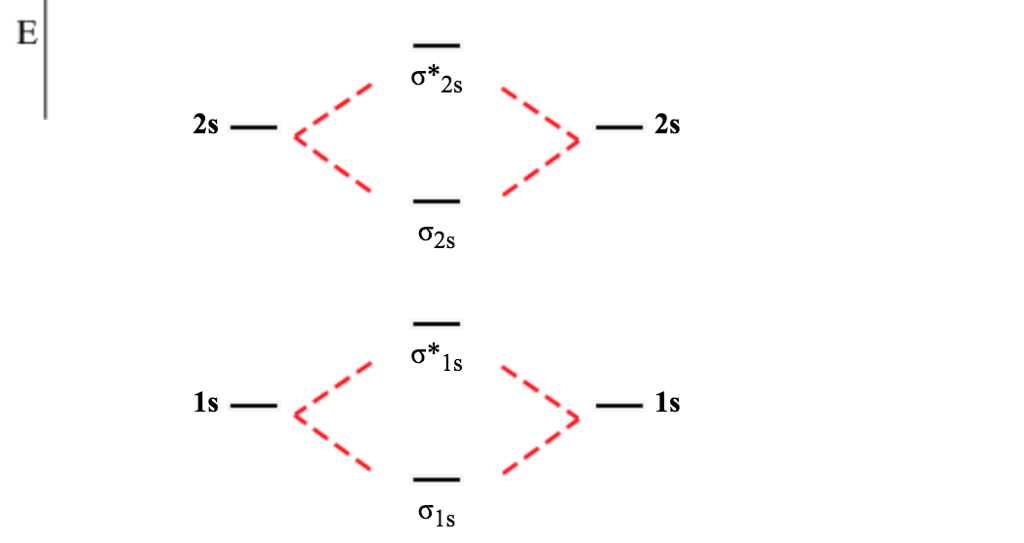
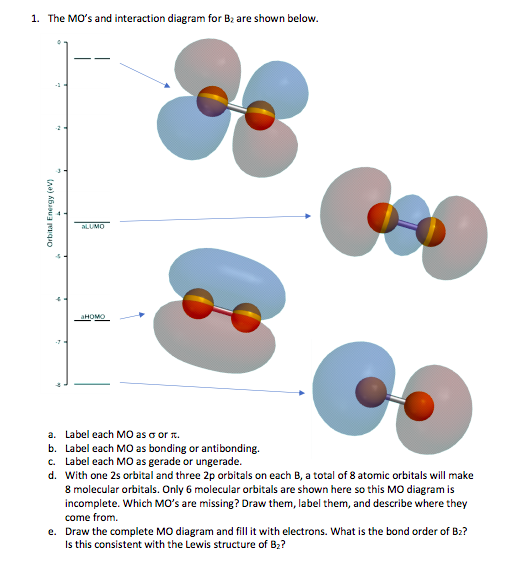
Before we get there it is worth while knowing a generic valence molecular orbital diagram where no s-p mixing occurs. This one pretty much applies to all main group elements heavier than nitrogen. The core orbitals, in case of lithium to neon these are the 1s orbitals, sodium to argon these are 1s, 2s, and 2p orbitals, are not included, as they ...
If a cos θ + b sin θ = m and a sin θ - b cos θ = n, then show that a2 + b2 = m2 + n2 #1 Free Secondary School Test Papers in Singapore (UPDATED ... Draw a molecular orbital diagram of N2 or O2 with magnetic Draw a molecular orbital diagram of N2 or O2 with magnetic behavior and bond order. Claim your FREE Seat in Vedantu Master Classes!
1 day ago · Molecular Orbital Theory and MO diagram of Dibromine (Br2) The MO diagram or Molecular Orbital diagram is an extension of the 3-dimensional molecular design and gives a better understanding of the structure of an atom. Molecular Diagram also reflects upon bond length, bond shape, bond energy, and the bond angle between 2 atoms.
B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3. In the formation of B2 molecule, three valence electrons of each boron atom i.e. 6 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
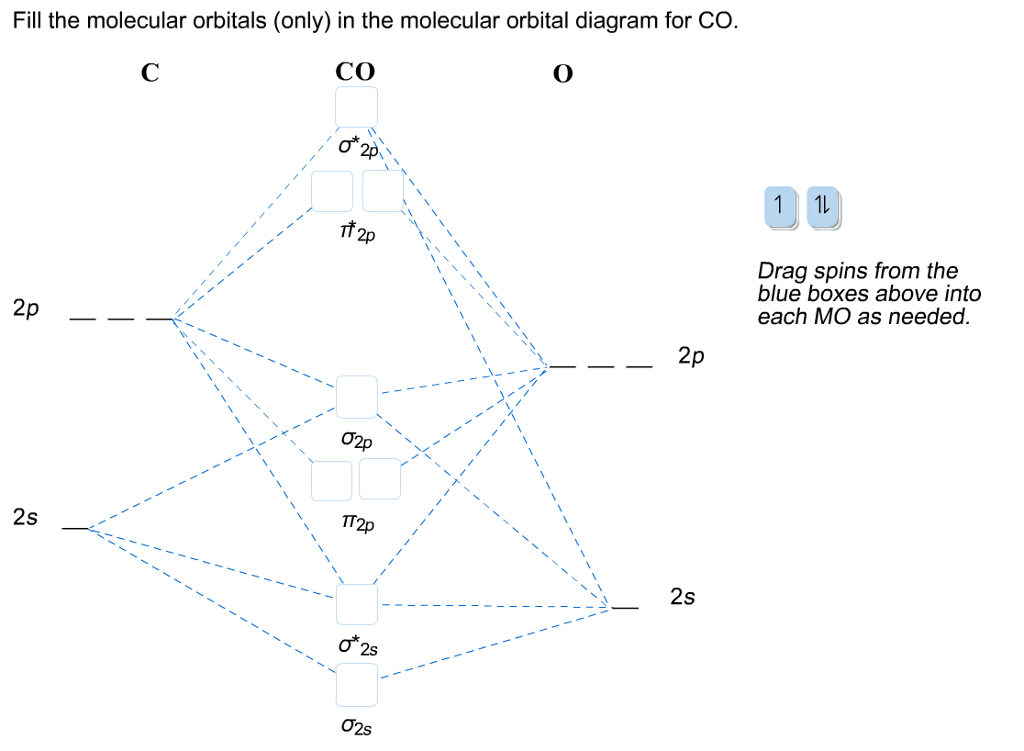
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence ...
FREE Answer to Question 1) By drawing molecular orbital diagrams for B2, C2, N2, O2, and F2, predict which...
molecular geometry = trigonal pyramidal. The molecular dipole moment is coincident with the 3-fold axis of the molecule and is directed towards the N. 1.47D Applications of Group Theory to the Physics of Solids The manner in which atomic orbitals overlap to form molecular orbitals is actually more complex than the localized examples given above.
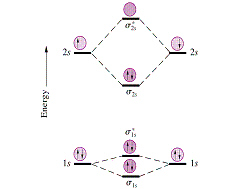
Answer (1 of 5): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To …
The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding electrons. ... Eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Li2, Be2, B2, C2, N2, ...
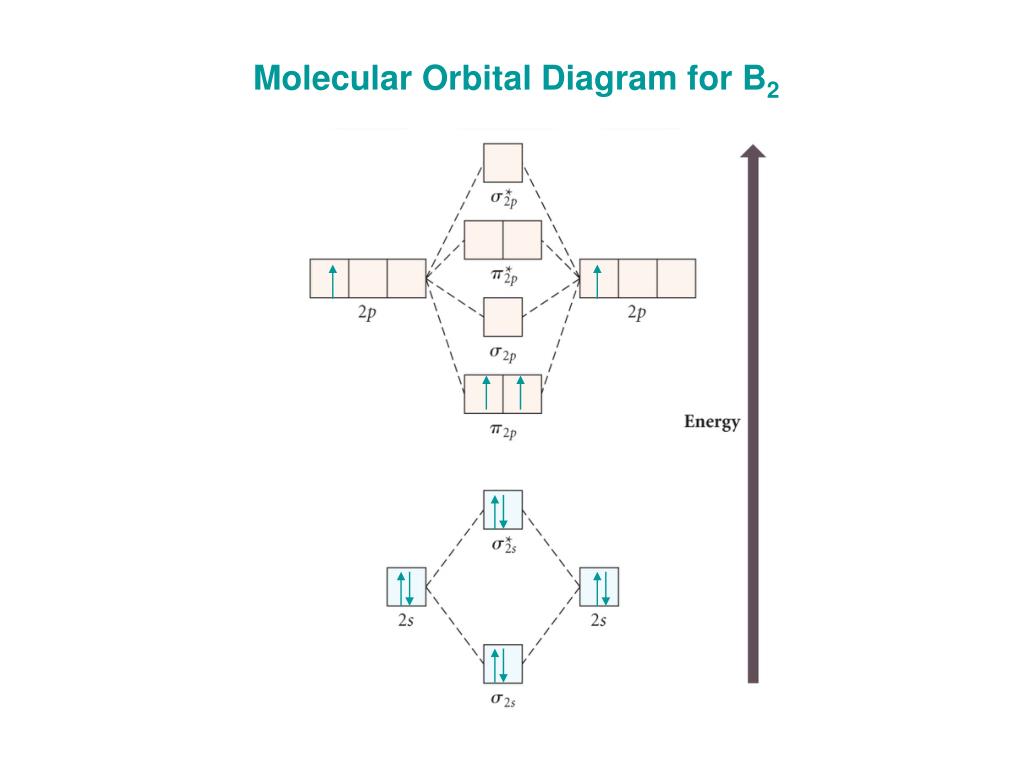
As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond.
Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero . This problem has been solved! See the answer See the answer See the answer done loading.
August 15, 2020 - This gives you the total number of electrons you will have to distribute among the molecular orbitals you form. For example consider B2 (each atom has an electron configuration of [He]2s22p), which has a total of 6 valence electrons. Draw a cartoon energy level diagram with lines for the valence ...
Jan 31, 2018 — To find the bond order of O2+ we can use the concept of Molecular Orbital Theory. In this method we have to count the number of molecules in the Bonding ...7 answers · 57 votes: O2 2- bond order = 1 O2 - bond order = 1.5 O2 bond order = 2 O2+ bond order = 2.5 O2 ...What is the molecular orbital diagram for O2- and ...5 answersMar 27, 2017How do I find the bond order of Cl2?
⇒ A homo-nuclear diatomic molecular orbital in which the same atoms combine together. example- N2, O2, B2, etc. ⇒ A hetero-nuclear diatomic molecular orbital in which different atoms combine together. example- CN, HF, NO, etc. Clearly, Cyanide (CN) lies in a hetero-nuclear diatomic molecular orbital as it contains two different atoms. Also, using the …
The molecular orbital diagram for B 2 molecule is as follows: We know that bond order is the difference between the number of bonds and the antibonds. Now, we have to calculate the bond order of B 2 molecule using the formula as follows: Bond order = 1 2 ( Number of electrons in BMO) − ( Number of electrons in ABMO) From the diagram, we can ...
This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. . × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. ...
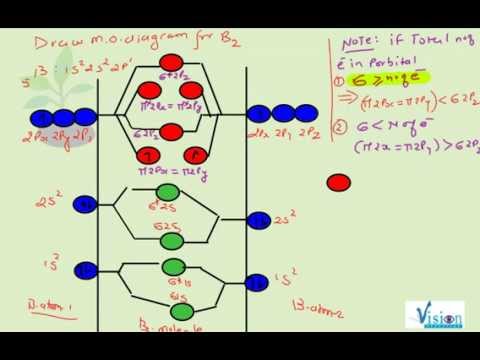
This video discusses how to draw the molecular orbital (MO) diagram for the B2 (boron) molecule. The bond order of the boron molecule is also calculated and ...
November 9, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
Bond order for N2 is 3; bond order for N2- is 2.5 and bond order for N2+ is 2.5. Need to understand molecular orbitals to see why this is the case. When the nitrogen atoms combine they form both S and P bonding and anti-bonding molecular orbitals.
Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape . There are two types of MO diagrams: Recall that the bonding MOs are those without an asterisk (e.g., σ1s), while the antibonding MOs are those with an asterisk (e.g., σ1s*).
January 18, 2017 - "BO" = 1/2 Boron atom is atomic number 5 in the periodic table, so it has five electrons. Thus, B_2 carries ten total electrons. The atomic orbitals each boron contributes consists of the 1s, 2s, and 2p. The ns orbitals combine to give a portion of the molecular orbital (MO) diagram like this: ...
18/08/2020 · A simplified Jablonski diagram listing the formation and decay ... B2, organic phosphors with an ... from a mechanistic perspective, tuning of the molecular orbital configurations based on the El ...
The valence molecular orbital diagram for Li2- is shown. The molecular orbital bond order for this species is equal to ____ and Li2- is _____stable than Li2. 1/2 ; less. the valence molecular orbital diagram for the anion B2- is given. Which of the following options correctly interpret this diagram? - B2- has a shorter bond than B2-The molecular orbital bond order is equal to 3/2. …
September 15, 2017 - Upon ionization one π electron ... and B2+ is formed, which has a one electron σ bond, instead of a π bond. It has been shown that a few carefully chosen VB configurations are enough to describe the bonding; with these structures, geometrical parameters as well as dissociation energies of these unusual molecular species are ...
Bonding; Question. Answers. Draw a molecular orbital diagram of ${N_2}$ or ${O_2}$ with magnetic behavior and bond order. Answer. … If a cos θ + b sin θ = m and a sin θ - b cos θ = n, then show that a2 + b2 = m2 + n2 With test papers, students are both exposed to more question types as well
September 15, 2016 - Board index Chem 14A Molecular Shape and Structure *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism) ... Hello, If there are an odd amount of electrons, (ex. B2+), how would we draw the atomic orbitals for this? There are an uneven amount of electrons, so which electrons would ...
Inorganic Chemistry (Atkins, Shriver).PDF
December 31, 2020 - So that no one else could suffer the same claustrophobic fate again. Molecular orbital diagram of b2. Use the molecular orbital diagram shown to determine which of the following are paramagnetic. It took me a full day to fix the mount figure out the wiring install magnets inside the old speedometer ...
Hey guys, So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons – # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond .
A) F2; B) F2^2+ C) Ne2^2+ D) O2^2+ E) F2^2-2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. 10 5 molecular orbital theory chemistry libretexts.
Orbital Interaction Theory of Organic ChemistryA Textbook of Inorganic Chemistry - Volume 1Molecular Orbitals and Organic Chemical ReactionsIntroduction to Molecular-orbital TheoryElectronic Absorption Spectra and Geometry of Organic MoleculesA Semi-empirical Mo Theory of Sigma Electron Systems. I. General
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
Answer to Molecular Orbital theory predicts that the B2 2- ion would have a bond order of ________. If one of the highest valence ...
.png)




















0 Response to "41 molecular orbital diagram b2"
Post a Comment