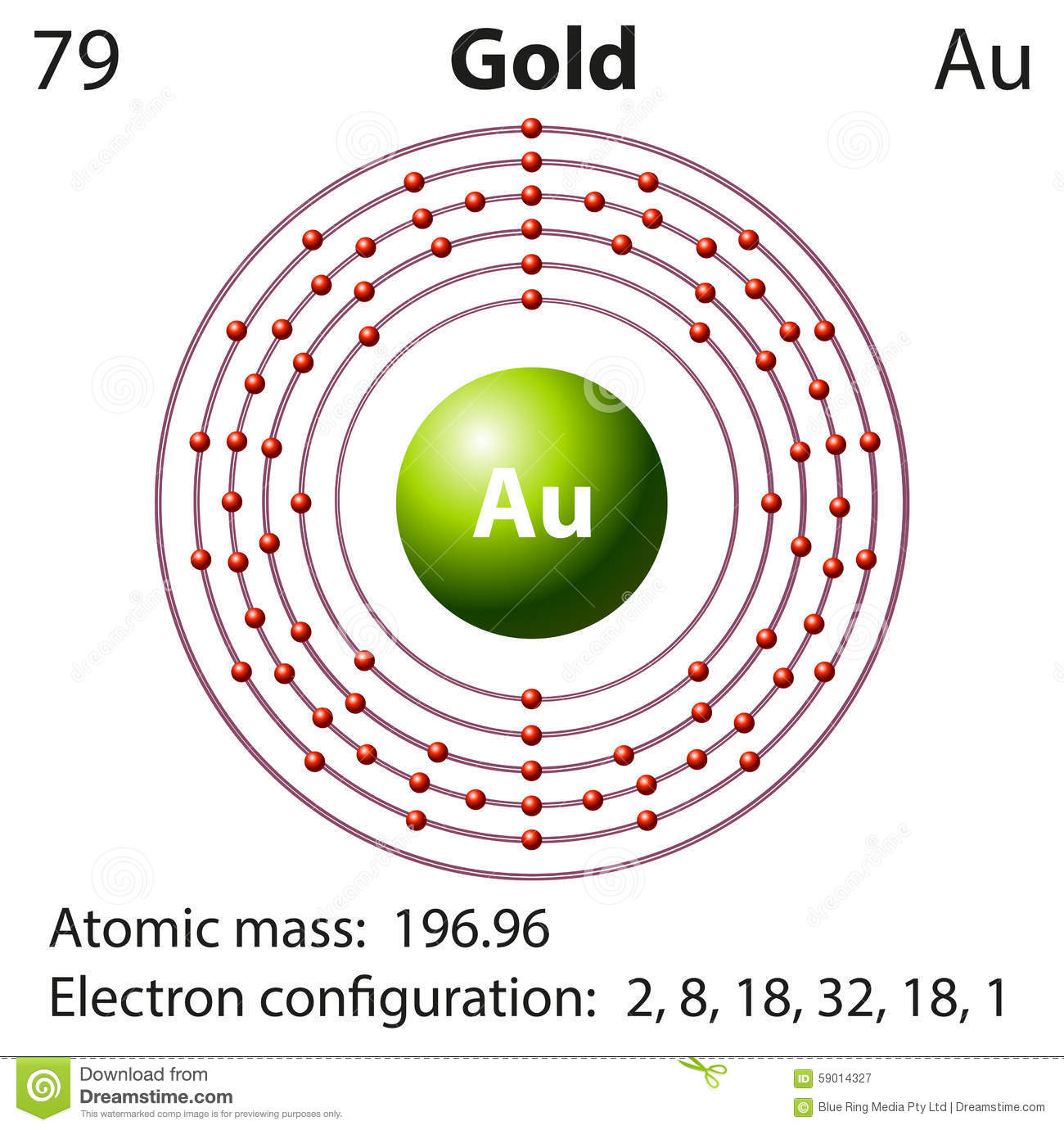
42 bohr diagram for gold
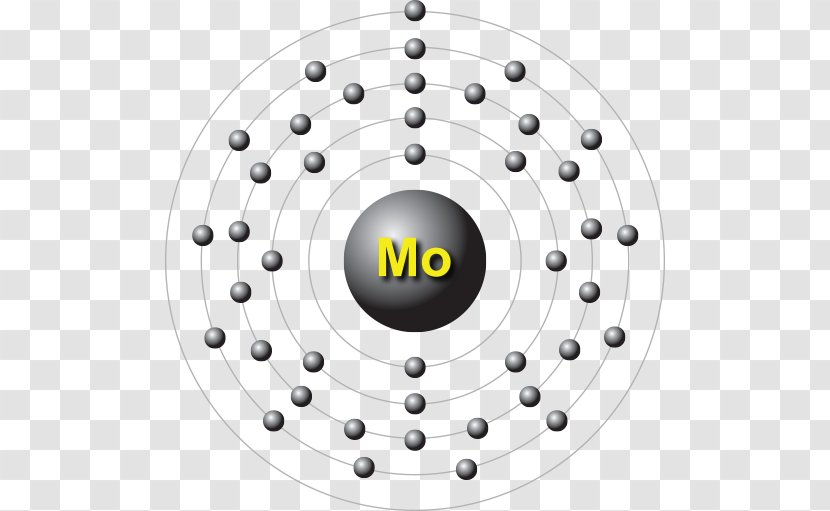
Gold (Au). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of gold-197 (atomic number: 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the solar system Joseph Larmor model (1897), the cubical model (1902 ...
Drawbacks Neil Bohr's Theory: Bohr's model was not able to define the effect of magnetic field and electric field on the spectra of atoms. The Bohr atomic model made correct predictions for smaller-sized atoms like hydrogen, but poor spectral predictions were obtained when larger atoms are considered.
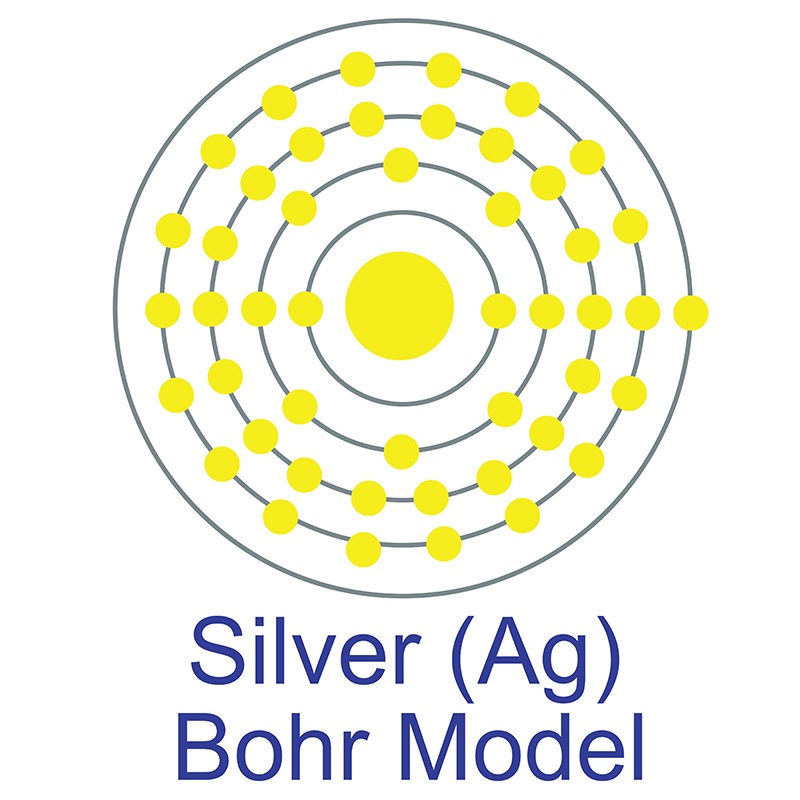
Bohr diagram for gold
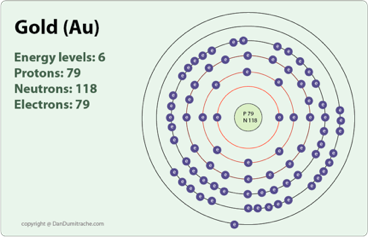
Name: Gold Symbol: Au Atomic Number: 79 Atomic Mass: 196.96655 amu Melting Point: 1064.43 °C (1337.5801 K, 1947.9741 °F) Boiling Point: 2807.0 °C (3080.15 K, 5084.6 °F) Number of Protons/Electrons: 79 Number of Neutrons: 118 Classification: Transition Metal Crystal Structure: Cubic Density @ 293 K: 19.32 g/cm 3 Color: Gold Atomic Structure and Bohr's Model Q6. Expansion of Bohr's Model Q7.Wave Mechanics Q8. Tunneling----- S-6.The X-ray Sun S-7.The Sun's Energy S-7A. The Black Hole at our Galactic Center LS-7A. Discovery of Atoms and Nuclei S-8.Nuclear Power S-9.Nuclear Weapons: The Atomic Nucleus Planck's law described the way hot matter in bulk radiated energy. To gain ... Supposedly around half of the world's supply of gold is stored in the United States Treasury Department's gold depository in Fort Knox Kentucky, which is considered to be one of the most secure buildings in the world. Additional Notes: Gold is poorly absorbed by the body. Gold-based anti-arthritics can cause liver and/or kidney damage. Gold Menu
Bohr diagram for gold. Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ... Answer: 1. Find the gold element on the periodic table. It's symbol is "Au" you'll find the gold's atomic number and atomic mass on the periodic table. 2. Determine the number of electrons. It's the same as the atomic number > 6 3. You need to notice the gold's row in thr periodic table. It's 6. ... Answer: 1. Find the gold element on the periodic table. It's symbol is “Au” you'll find the gold's atomic number and atomic mass on the periodic table. 2. Determine the number of electrons. It's the same as the atomic number > 6 3. You need to notice the gold's row in thr periodic table. It's 6. ... Ex. Consider the element gold. Its symbol is Au. Its mass number is 197 and its atomic number is 79. 197 Wrutten in standard atomic notatuon it becomes: 79 Write the standard atomic notation for germanium, uranium, and cobalt. Modeling Atoms with Bohr Diagrams Atoms are so that in order to study them, we need to create of electrons in the ...
Bohr's Model. Neils Bohr knew about all of these facts, and in the early part of the century was collaborating with Rutherford. He also knew about the existence of line spectra from chemical elements; a document on this topic may be found here. He was struggling to make sense of all of this. The diagram is called the bohr diagram. The Lewis Dot Diagram shows how many electrons an element has on it's outer valiance shell. Gold only has one. Atomic number: 79 - The atomic number is how many protons there is in an element. ... Electrons: A neutral atom of gold has 79 electrons that orbit around the protons and neutrons. Gold has very ... Part A: The Bohr Model Using Standard Atomic Notation . Ex. Consider the element gold. Its symbol is Au. Its mass number is 197 and its atomic number is 79. Written in standard atomic notation it becomes: 1 79 97. Au. Write the standard atomic notation for germanium, uranium, and colbalt. Modeling Atoms with Bohr Diagrams Bohr found that an electron located away from the nucleus has more energy, and electrons close to the nucleus have less energy. Postulates of Bohr's Model of an Atom In an atom, electrons (negatively charged) revolve around the positively charged nucleus in a definite circular path called orbits or shells.
Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of Silicon (Si) 2, 8, 4: 15: Bohr ... How to draw the Bohr-Rutherford Diagram for Germanium. The order of filling makes Bohr-Rutherford Diagrams for Elements beyond #20 (Calcium) tough. 2 in th... Bohr's model of the hydrogen atom explains the emission and absorption spectra of atomic hydrogen and hydrogen-like ions with low atomic numbers. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.
Valance Electrons, Quantum Numbers and Bohr Model Diagram. Valance electrons are the electrons on the outermost ring. They can react with other elements during chemical reactions. Gold's dot diagram to the left contains one valance electron. The four quantum numbers for the last electron in gold's orbital diagram are 5,2, 2, -1/2.
H ear this out loudPauseAnswer: Bohr found experimental evidence for his atomic model by studying line spectra. Line Spectra is plural of a line spectrum and a line spectrum is an emission of light, sound, or other radiation, composed of a number of discrete frequencies or energies (a spectrum).
download bohr rutherford diagram of gold pdf Being able to help visualize atoms has allowed scientists to gain a better understanding of why reactions occur and compounds form. 2 oct 2018 orbital diagram for gold fx x one piece orbital diagram for au orbital gold electron configuration diagram diagram for iron orbital diagram for ca orbital ...
Bohr Diagrams and Lewis Dot Structures What you've already learned in class and from readings You learned that Electrons can exist in different energy levels You learned that the # of Electrons in an atom are equal to the # of Protons in an atom You learned that the # of Valence Electrons are the outermost Electrons of an Atom What You're about to learn How to draw the Electrons around an ...
how to determine the charge of an atom by looking at the bohr model. how to determine the charge of an atom by looking at the bohr model.
What experiments led to the Bohr model? Rutherford experiment with alpha particles shot at a thin gold foil resulted in the Rutherford model of the atom (Orbital Model). This model depicted an atomic model with nearly all its mass, and positive charge, in a central nucleus about 10,000 times smaller than the atom itself.
In his calculations, Bohr came across a number now called the "Bohr radius". This number is 5.29 x 10-11 m and is a.The maximum radius of the hydrogen atom. b.The minimum radius of the hydrogen atom. c.The average radius of the hydrogen atom. d. The minimum radius of atoms of all elements.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Rutherford and Bohr describe atomic structure 1913. Photo: Niels Bohr's research notes for his new atomic theory. ... at a sheet of gold foil only 1/3000 of an inch thick, and tracing the ...
Bohr model for Platinum Bohr Model, Electric Fan, Picture Books, Art Photography, . Science project, platinum atom model by Issac Yescas Neon Atom Model. Platinum. Au. Gold. Hg. Mercury. Ti A Bohr Diagram shows a nucleus surronded by orbits of electrons. Bohr model in art (1 C, 25 F) 1 H Bohr wiringall.com × ; 8 KB.
Bohr Model Challenge. Model the first 18 elements, starting with Helium using the Bohr model: Bohr Model Element # of valence electrons Period Group Metal, nonmetal or metalloid
Niels Henrik David Bohr (Danish: [ˈne̝ls ˈpoɐ̯ˀ]; 7 October 1885 - 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922. Bohr was also a philosopher and a promoter of scientific research.. Bohr developed the Bohr model of the atom, in which he proposed ...
Supposedly around half of the world's supply of gold is stored in the United States Treasury Department's gold depository in Fort Knox Kentucky, which is considered to be one of the most secure buildings in the world. Additional Notes: Gold is poorly absorbed by the body. Gold-based anti-arthritics can cause liver and/or kidney damage. Gold Menu
and Bohr's Model Q6. Expansion of Bohr's Model Q7.Wave Mechanics Q8. Tunneling----- S-6.The X-ray Sun S-7.The Sun's Energy S-7A. The Black Hole at our Galactic Center LS-7A. Discovery of Atoms and Nuclei S-8.Nuclear Power S-9.Nuclear Weapons: The Atomic Nucleus Planck's law described the way hot matter in bulk radiated energy. To gain ...
Name: Gold Symbol: Au Atomic Number: 79 Atomic Mass: 196.96655 amu Melting Point: 1064.43 °C (1337.5801 K, 1947.9741 °F) Boiling Point: 2807.0 °C (3080.15 K, 5084.6 °F) Number of Protons/Electrons: 79 Number of Neutrons: 118 Classification: Transition Metal Crystal Structure: Cubic Density @ 293 K: 19.32 g/cm 3 Color: Gold Atomic Structure

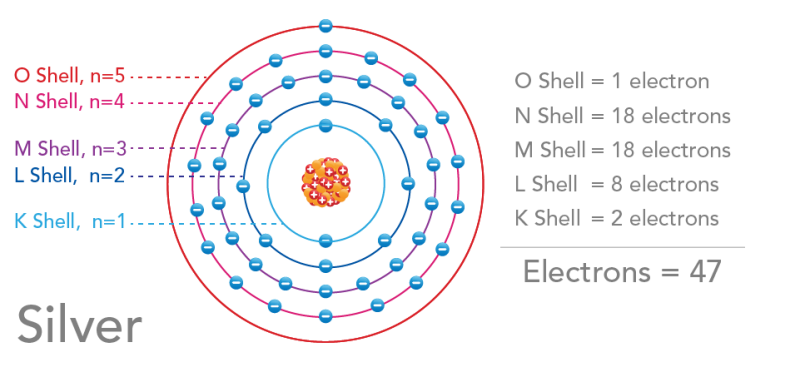
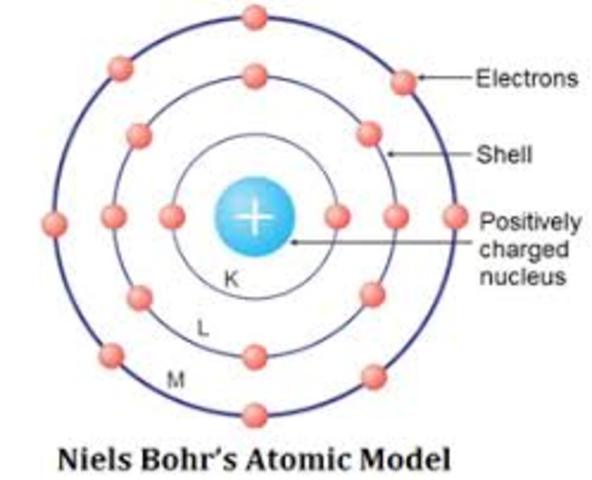
:max_bytes(150000):strip_icc()/radiumelectronshell-58b600ea3df78cdcd83c930f.jpg)




![[DIAGRAM] Gold Bohr Diagram](https://blogger.googleusercontent.com/img/proxy/AVvXsEhBCqm2HVEi6XxB6E3PxKMSqdc9YF483DDdpovqi_2WKjACY900_7zd0pW4kXgwooL7ZHbWcMQRh_rv1pG4_YUyAipOt6mCHdj9Mm8rLzm2sTnUdAGO2R-Hn16molzNyAs1AXQpE0gAI9bMoA3HbP1W0_RQM4WnNt3AEKX8-3YQprHv5ylxFlBXimiI5w=w1200-h630-p-k-no-nu)











_enhanced_Bohr_model.png/120px-79_gold_(Au)_enhanced_Bohr_model.png)



0 Response to "42 bohr diagram for gold"
Post a Comment