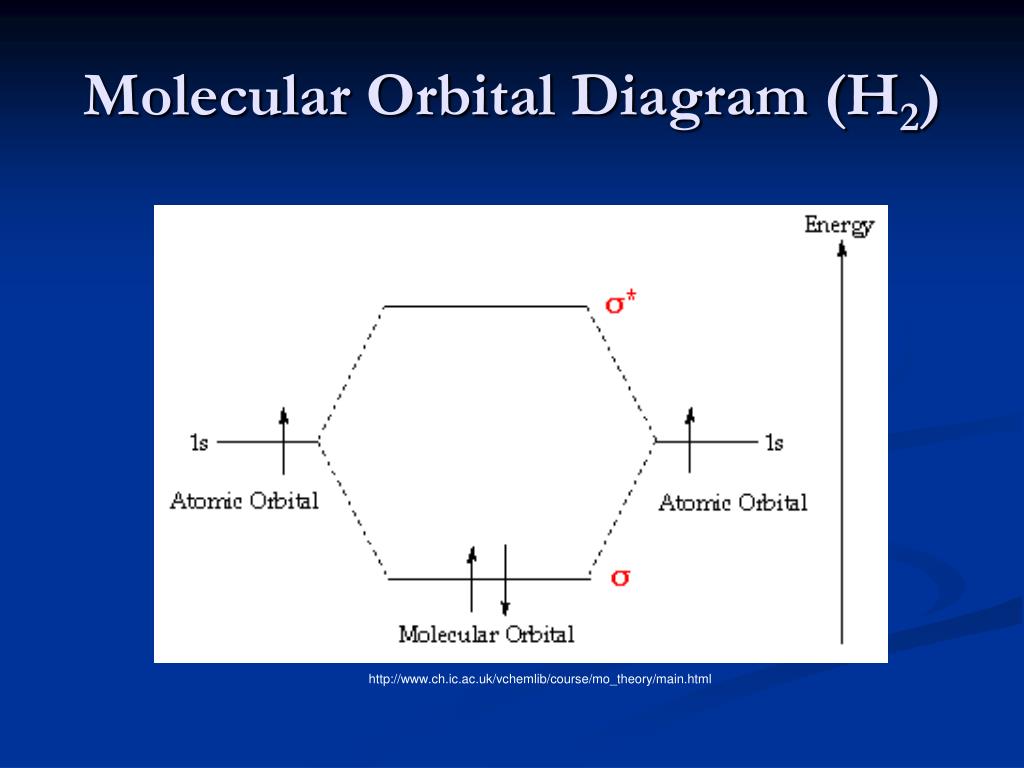
37 Molecular Orbital Diagram H2
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable. Molecular Orbital Theory - Chemistry | Socratic Molecular orbital theory is a method for determining molecular structure. It describes electrons as moving under the influence of the nucleus and not Molecular Orbital (MO) theory better explains the properties of more complex molecules. MO theory explains the partial bonds of NO₃⁻ without using...
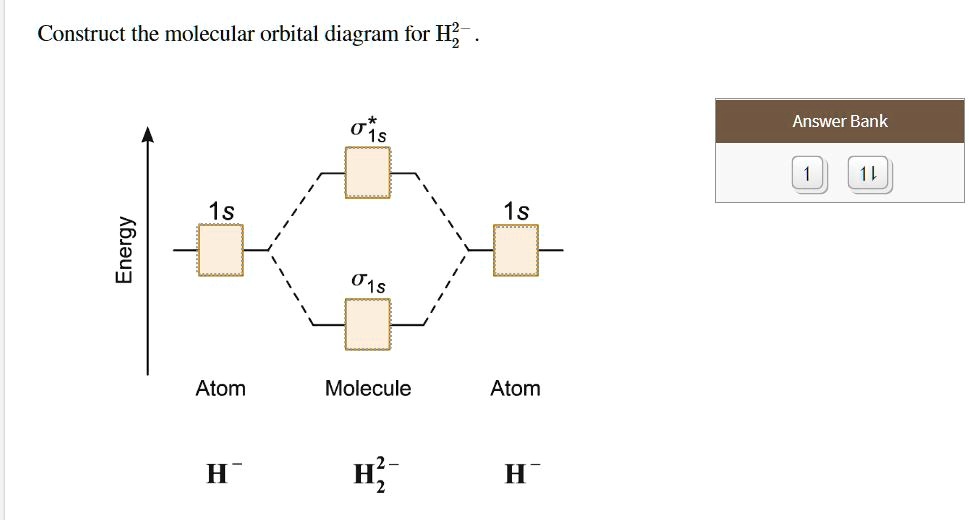
Solved Construct the molecular orbital diagram for... | Chegg.com Transcribed image text : Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: 0 0.5 1 1.5 2 Click within the blue boxes to add electrons.
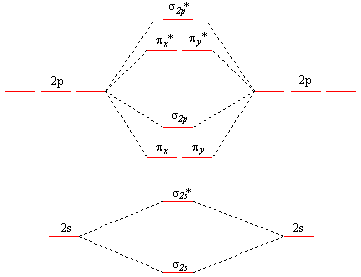
Molecular orbital diagram h2
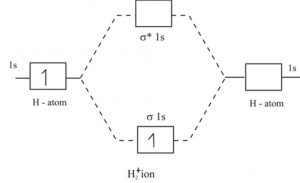
Energy level diagram for Molecular orbitals - Chemical Bonding and... 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding Greater value of bond order for H2 molecule than H2+ ion shows that two H2 molecule is more stable than H2+. MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row tikz pgf - Molecular orbital diagram for CH2 - TeX - LaTeX Stack... Learn more. Molecular orbital diagram for CH2. I'm trying to draw an MO diagram for CH2 by mimicking this MO diagram (excluding the orbital images), but things don't look like the way I want it to be.
Molecular orbital diagram h2. PDF lecture_6 Molecular orbital 'resembles' the atomic orbital to which it lies closest in energy. Always break MO diagrams down into components based on symmetry. Walsh diagrams summarise changes in MO diagram wrt structure note a combination of first and second order effects. Chapter 2 - Molecular orbital theory Use molecular orbital theory to predict molecular geometry for simple triatomic systems • Rationalize molecular structure for several specific systems in H3: From linear to bent H3: From linear to bent Walsh diagram H3+ vs H3-What does this suggest about the molecular geometry of H3+ vs. H3... Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations The geometric mean of the H2 and Li2 bond energies is 213 kJ/mole, so it appears that the lithium hydride molecule is 30 kJ/mole more stable... [Expert Answer] Draw the molecular orbital diagram for... - Brainly.in Molecular orbital diagram and bond order of fluorine molecule. Fluorine molecule is formed by the combination of atomic orbitals of two fluorine atoms, each having nine electrons, thus making 18 electrons. These 18 electrons are filled in various molecular orbitals, in the increasing order of their...
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major Valence Bond Theory proposes that electrons are localized between two atoms. On the other hand, Molecular Orbital Theory visions the electrons of a... Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. We begin our discussion of molecular orbitals with the simplest molecule, H2, formed from two isolated hydrogen atoms, each with a 1s1 electron configuration. 1.3.2 Molecular Orbital Energy Level Diagram for... | Quizlet what electrons do we have to look at for the molecular orbital energy level diagrams with period 2 elements. -1s are core electrons so not that -Li2 and Be2 will be the same as H2 and He2 just the electrons will be from the 2s orbital. -2 p orbitals need to be considered for B to F.
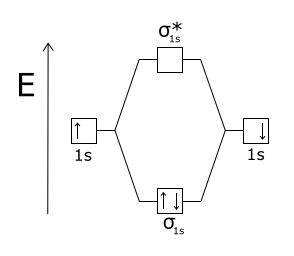
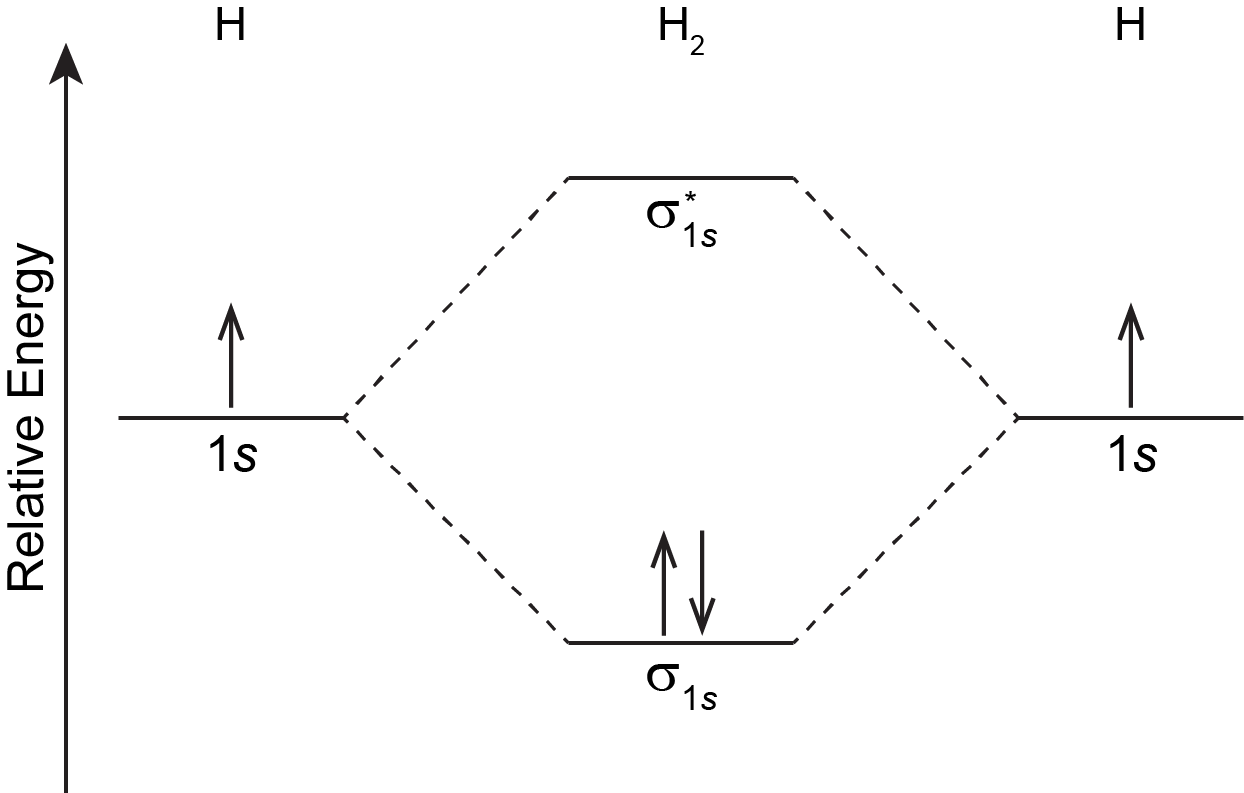
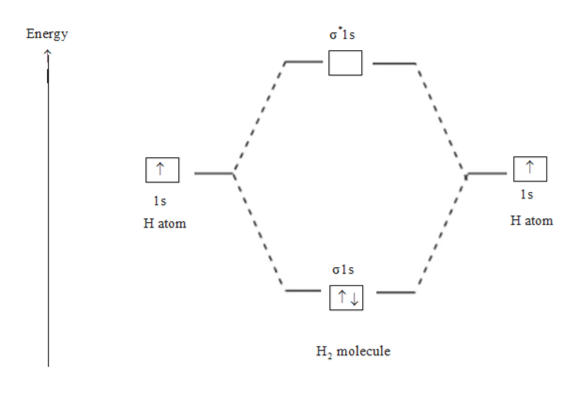
Wikizero - Molecular orbital diagram Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Molecular Orbital Diagram of H2, He2, Li2 and... - YouTube Similarly we can draw the molecular orbital diagram of He and find the number of covalent bond formed between two helium atoms. He has two electrons with electronic configuration 1s2. 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 8.35 The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Figure 14: The molecular orbital energy-level diagram for diatomic... Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the bonding (1σ) and antibonding (2σ) molecular orbitals they form.
Figure 7. Molecular orbital energy level diagrams for HS − and H 2 S. These data support soluble molecular clusters in ... In this study, we measured the sulfur isotope fractionation between total dissolved sulfide and gaseous H_2S at 20.6±0.5°C over the pH range from 2 to 8, and calculated the equilibrium isotope effects associated with deprotonation of dissolved H_2S.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Molecular Orbital Theory | Brilliant Math & Science Wiki Molecular orbital theory approximates the solution to the Schrödinger equation for a molecule. Energy calculations are used to test the validity of the Energy level diagrams are used to determine whether or not a molecule will be stable. The atomic orbitals of the two atoms are drawn on the sides of the...
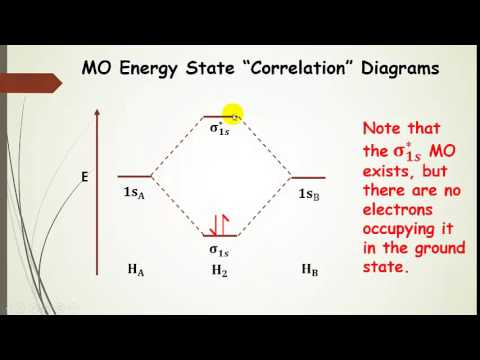
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. Because the two electrons in an H2 molecule are in a bonding orbital, the bond order is one. We conclude that the H2 molecule would be stable, and we...
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
8.4 Molecular Orbital Theory - Chemistry The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 9. The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms.
Is H2 a viable molecule for the molecular orbital theory? - Quora The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
File:H2O-MO-Diagram.svg - Wikimedia Commons Added orbital diagrams for molecular orbitals. Moved 2s orbital higher in energy as that MO has more hydrogen admixture. The following other wikis use this file: Usage on en.wikipedia.org. Molecular orbital diagram.
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of molecular orbitals (MOs) (σ, π and g, u) - Homonuclear diatomic MO diagrams - mixing of different AO's - More complex molecules (CO, H2O ….)
Molecular Orbital Theory The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. Placing an electron in this orbital therefore stabilizes the H2 The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the...
Answered: Construct the molecular orbital diagram… | bartleby Science Chemistry Q&A Library Construct the molecular orbital diagram for H2−. Identify the bond order.
Molecular Orbital Theory | Boundless Chemistry In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; this This MO diagram depicts the molecule H2, with the contributing AOs on the outside sandwiching the MO. The bonding level (lower level) is completely...
tikz pgf - Molecular orbital diagram for CH2 - TeX - LaTeX Stack... Learn more. Molecular orbital diagram for CH2. I'm trying to draw an MO diagram for CH2 by mimicking this MO diagram (excluding the orbital images), but things don't look like the way I want it to be.
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Energy level diagram for Molecular orbitals - Chemical Bonding and... 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding Greater value of bond order for H2 molecule than H2+ ion shows that two H2 molecule is more stable than H2+.





















0 Response to "37 Molecular Orbital Diagram H2"
Post a Comment