37 Square Planar Molecular Orbital Diagram
Harry B. Gray and C. J. Ballhusa - Molecular Orbital Theory For... A Molecular Orbital Theory for Square P a a Metal Complexes lnr. BY HARRY GRAY B. AND C. J. BALLHAUSEN' RECEIVED JULY 25, 1962 The bonding in square planar metal complexes is described in terms of molewlar orbitals. The relative single electron molecular orbital energies are... Tetrahedral vs. Square Planar Complexes - Chemistry LibreTexts In square planar molecular geometry, a central atom is surrounded by constituent atoms, which form the corners of a square on the same plane. The CFT diagram for square planar complexes can be derived from octahedral complexes yet the dx2-y2 level is the most destabilized and is left unfilled.
Orbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three ...
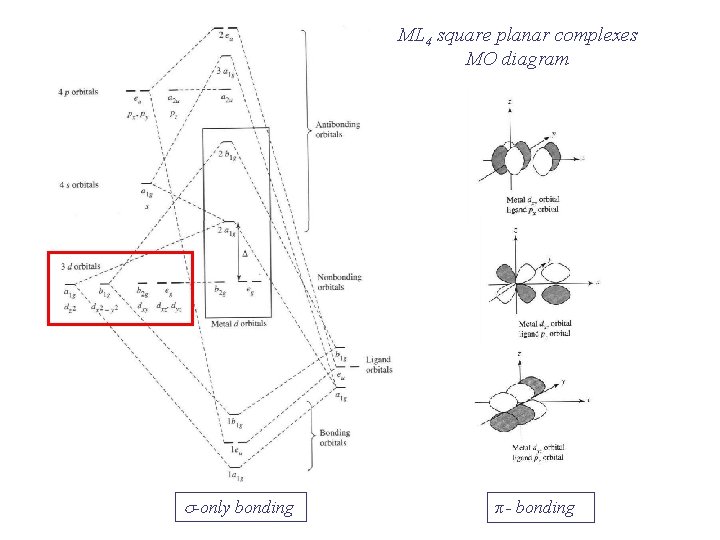
Square planar molecular orbital diagram
Metal-Ligand and Metal-Metal Bonding Core Module 4 RED Why complexes form. 18-electron rule. Recap of molecular orbital theory. s-donor ligands (hydride complexes). Construction and interpretation of octahedral ML 6 molecular orbital energy diagram Lecture 3: p-acceptor ligands, synergic bonding, CO, CN-, N 2, Lecture 4: Alkenes and alkynes. Dewar-Duncanson-Chatt model. Lecture 5: M(H 2) vs M(H) 2 ... PDF M A Walsh diagram for an B2A or AB2 molecule is constructed by considering how the composition and energy of each molecular orbital changes as the bond angle changes from 90o to 180o. 2.13: Molecular Orbital Energy Level Diagram in a Square Planar Complex. Square Planar Molecular Geometry: study guides and answers on... Square Planar Molecular Geometry Square Pyramidal Molecular Geometry Ball And Stick Model Ground State Electron Configuration Double And Triple Bonds. Square Planar Molecular Geometry Lewis Dot Diagram Electron Pair Geometry Opposite Charges Repel Nonpolar Covalent Bonds.
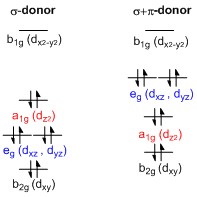
Square planar molecular orbital diagram. Part 9(e): ligand field theory (mo diagram square... In this video, I have explained the detailed molecular orbital diagram for square planar complexes. Formation of sigma lgo and pi lgo have been discussed in... Square planar molecular geometry - WikiMili, The Free Encyclopedia The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain A general d-orbital splitting diagram for square planar (D4h) transition metal complexes can be derived from the general octahedral (Oh) splitting... File:D-orbital splitting diagrams of square planar complexes.jpg... D-orbital_splitting_diagrams_of_square_planar_complexes.jpg (197 × 197 pixels, file size: 10 KB, MIME type: image/jpeg). English: Representative d-orbital splitting diagrams for square planar complexes featuring σ-donor (left) Usage on en.wikipedia.org. Square planar molecular geometry. Crystal field theory - Wikipedia Crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, usually d or f orbitals, due to a static electric field produced by a surrounding charge distribution (anion neighbors). This theory has been used to describe various spectroscopies of transition metal coordination complexes, in particular optical spectra (colors).
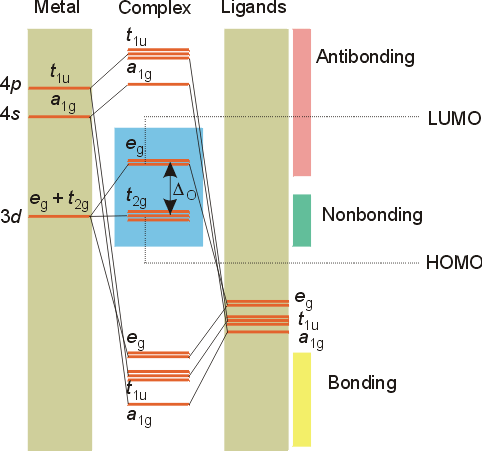
Benzene Lewis Structure, Molecular Geometry, Hybridization ... 12-02-2022 · By drawing molecular orbital diagrams of a molecule, one can predict the stability, bond order, and magnetic character of a molecule. Now, let us have a look at the MO diagram of benzene. The p-orbitals on each carbon atom could overlap to form six molecular orbitals, three bonding orbitals (ψ1 to ψ3) and three antibonding orbitals (ψ4 to ψ6) as shown in the figure. Molecular Orbital Theory – Octahedral, Tetrahedral or ... In square-planar complexes, the molecular orbitals created by coordination can be seen as resulting from the donation of two electrons by each of four σ-donor ligands to the d-orbitals on the metal. The molecular orbital energy level diagram for σ-bonding in square-planar complexes can be shown as Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. d-Metal Complexes - University of Massachusetts Lowell In this case, the d z 2 orbital drops even lower in energy, and the molecule has the following orbital splitting diagram. As a result of these distortions, there is a net lowering of energy (an increase in the ligand field stabilization energy) for complexes in which the metal has a d 7 , d 8 , or d 9 configurations, and thus electrons would occupy the upper e g set if an octahedral …
Molecular orbital diagram derived from the TD-DFT calculations. Square-planar high-spin Fe(II) molecular compounds are rare and the only three non-macrocyclic or sterically-driven examples reported share a common FeO4 core. Using an easily modifiable pincer-type ligand, the successful synthesis of the first compound of this type that breaks the FeO4 motif was... HYBRIDIZATION IN SQUARE PLANER COMPLEXS - ppt video online... 33 SQUARE PLANER MOLECULE GEOMETRY Idealized structure of a compound with square planar coordination geometry. The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds .As... How can we tell if a bond shape will be tetrahedral or square planar? MnO4's square planar shape is an average. Less common are some molecules of platinum-group metals Square planar molecular geometry - Wikipedia. The requirement for 8 electrons in d orbitals comes from the orbital splitting diagram for a square planar complex. This diagram is shown below. PDF Organometallic Chemistry Solution: The molecular orbital diagram is similar to that for O2, shown in Figure 2-4. The peroxide ion has two more antibonding electrons than neutral Molecular Orbitals of Square Planar Complexes (Only σ donor and π acceptor interactions. 4L are shown.) be significantly more stable than an...
PDF Lecture 1 Lecture 6: ML6 molecular orbital energy diagrams incorporating p-acceptor and p-donor ligands. Square-planar Pt(II) complexes have been studied most extensively: suitable timescale In an associative mechanism BOTH bond-breaking and bond-making can be important
A Molecular Orbital Theory for Square Planar Metal Complexes A detailed theoretical analysis of the synthesis and characterisation of two series of isoleptic and isoelectronic (d8) compounds revealed that the filled dz2 orbital is the HOMO in the case of Pt(ii), but is buried in the lower energy levels in the cases of Au(iii).
Molecular orbitals square planar complex - Big Chemical Encyclopedia Molecular Orbitals for Square Planar Complexes.— Figure 1 shows a square planar complex in a The general molecular orbital energy level schemes arrived at for square planar complexes are An energy diagram for the molecular orbitals of a square-planar molecule of formula ML4 (L = ligand...
Square planar molecular geometry - Wikipedia The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corners.
Square planar molecular orbital diagram Assortment of ge refrigerator wiring diagram. A wiring diagram is a simplified standard pictorial depiction of an electric circuit. It reveals the elements of the circuit as simplified forms, and the power and also signal connections between the tools.
CP SB #27 - Square planar molecular geometry? What is the max... Is the reason why these transition metal cations can form square planar geometry due to the fact that they have a d orbital which can expand to hold more than 4 1.) Yes, d-orbitals allow the formation of more complicated bonding geometries than just s or p orbitals. Square planar (and commonly trigonal...
PDF Molecular Geometry and Bonding Theories Chapter 9 CHEMA1301 The orbital diagram for a ground-state Be atom is: Because it has no unpaired electrons, the Be atom in its trigonal-planar molecular Notice that an unfilled 2p. agetoommiectoryrboitfaBlFr3e.mains. Molecular orbital theory correctly predicts that hydrogen forms diatomic molecules but helium does...
PDF lecture_6 Square planar complexes e.g [Ni(CN)4]2-, [PtCl4]2-Symmetry analysis (σ-only). ∆o. 18 vs 16-electron rules. which orbital is not being used? Walsh diagram for D4h Td. D4h Td n.b steric factors always favour Td (angles 109.5). Typical bond lengths.
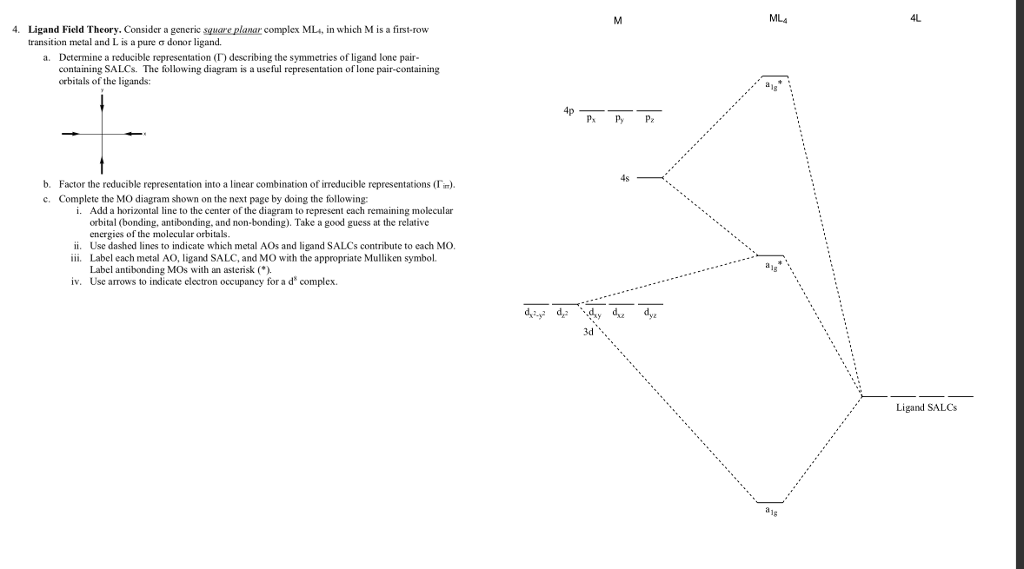
PDF Problems2_Square_planar_MO_diagram • square planar complexes can be derived by removing the axial ligands from an octahedral complex. x2+y2, z2 • determine the molecular shape: square planar. • I haven't been adding annotations, but describing orbital overlaps and energies in the text, in an exam you would be...
inorganic chemistry - How can I build the molecular orbital diagram of... I have to build the molecular orbital for the $\ce{[Cu(NH_3)_4]^{2+}}$ complex. I know I have to use the characters table, the irreducible representations The problem is as you state that the complex you wish to determine does not have a square-planar geometry (nor is it a tetracoordinated copper) so...
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... CD contains interactive energy diagrams. • Molecular is planar. • Three directions each at 120o. Æ mix s with 2 p orbitals. • Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation (these are not MO).
PDF M10_TRO5187_04_SE_C10_426-483_annod.indd Square planar. For Practice 10.2 Predict the molecular geometry and bond angle of ClNO. We can represent the molecular orbital energy diagram with a molecular orbital electron configuration (which is analogous to the electron configurations we wrote for elements in Section 8.3)
8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O2. Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here.
Molecular orbital - Infogalactic: the planetary knowledge core In chemistry, a molecular orbital (MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region.
Tetrahedral and Square Planar Complexes | Introduction to Chemistry In square planar molecular geometry, a central atom is surrounded by constituent atoms, which form the corners of a square on the same plane. CFT energy diagram for square planar complexesNotice how the dx2 - y2 orbital is unfilled.
Square Planar Molecular Geometry: study guides and answers on... Square Planar Molecular Geometry Square Pyramidal Molecular Geometry Ball And Stick Model Ground State Electron Configuration Double And Triple Bonds. Square Planar Molecular Geometry Lewis Dot Diagram Electron Pair Geometry Opposite Charges Repel Nonpolar Covalent Bonds.
PDF M A Walsh diagram for an B2A or AB2 molecule is constructed by considering how the composition and energy of each molecular orbital changes as the bond angle changes from 90o to 180o. 2.13: Molecular Orbital Energy Level Diagram in a Square Planar Complex.
Metal-Ligand and Metal-Metal Bonding Core Module 4 RED Why complexes form. 18-electron rule. Recap of molecular orbital theory. s-donor ligands (hydride complexes). Construction and interpretation of octahedral ML 6 molecular orbital energy diagram Lecture 3: p-acceptor ligands, synergic bonding, CO, CN-, N 2, Lecture 4: Alkenes and alkynes. Dewar-Duncanson-Chatt model. Lecture 5: M(H 2) vs M(H) 2 ...





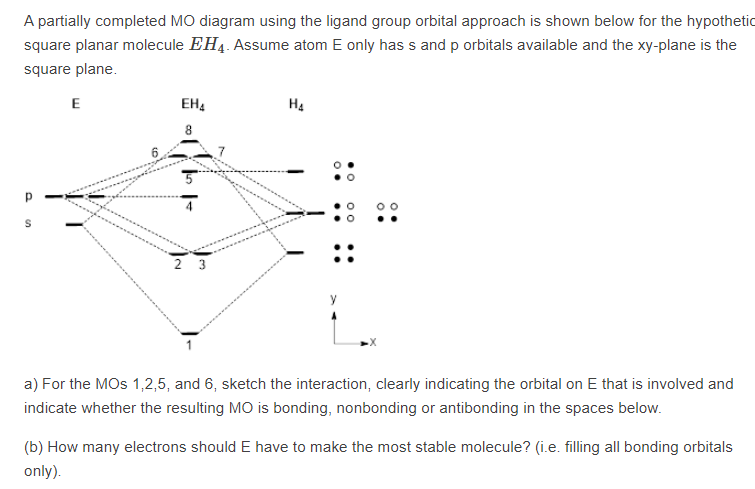


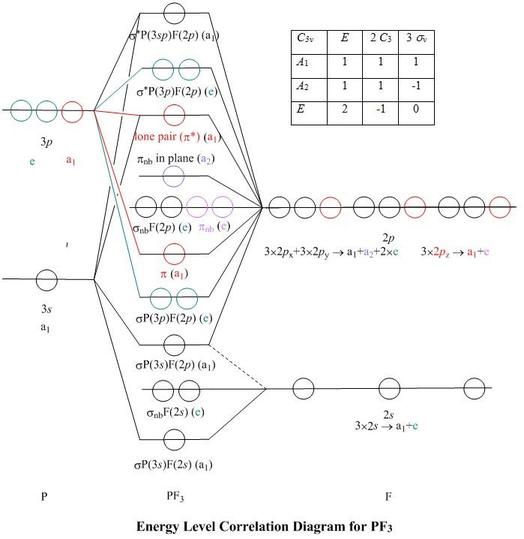
![d-orbital energy levels in planar [M II F 4 ] 2− , [M II (NH ...](https://pubs.rsc.org/image/article/2020/DT/d0dt02022b/d0dt02022b-f1_hi-res.gif)


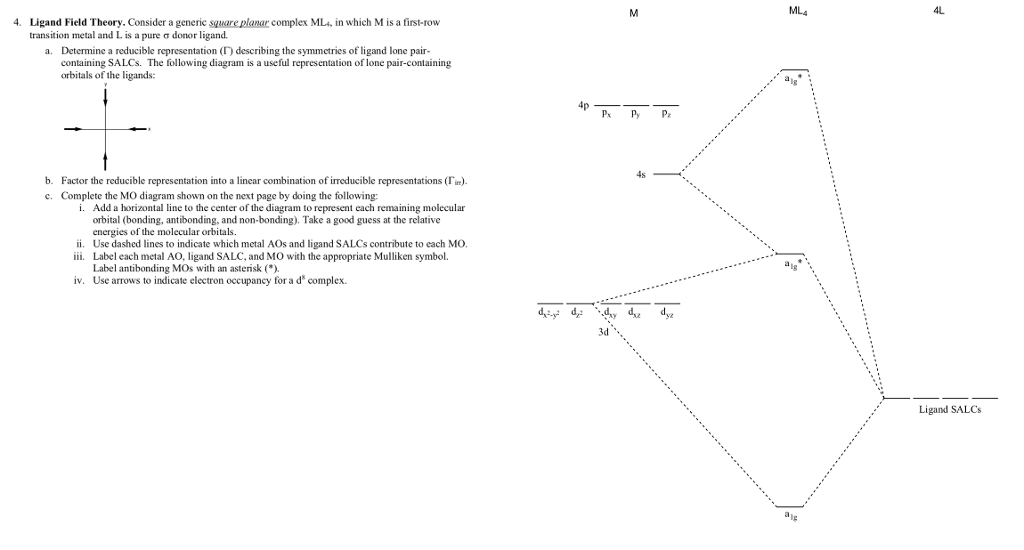


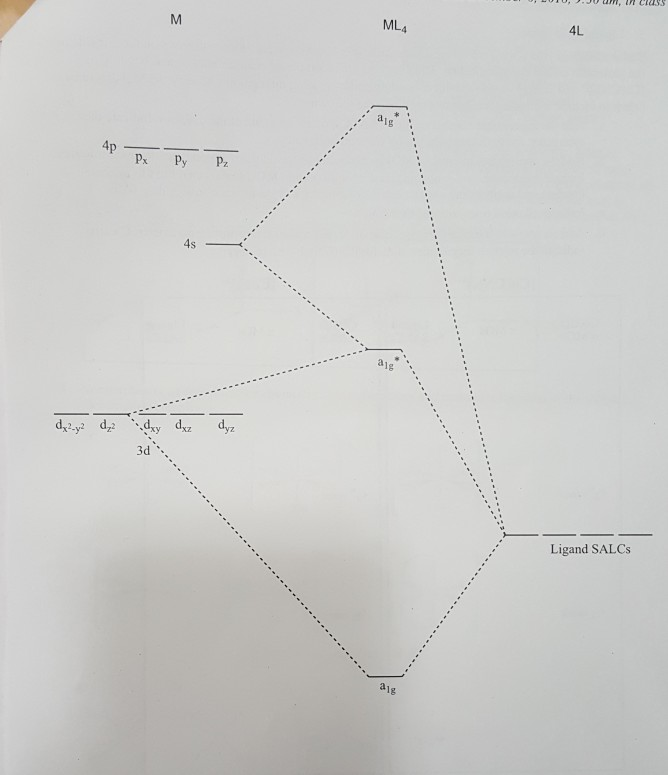


0 Response to "37 Square Planar Molecular Orbital Diagram"
Post a Comment