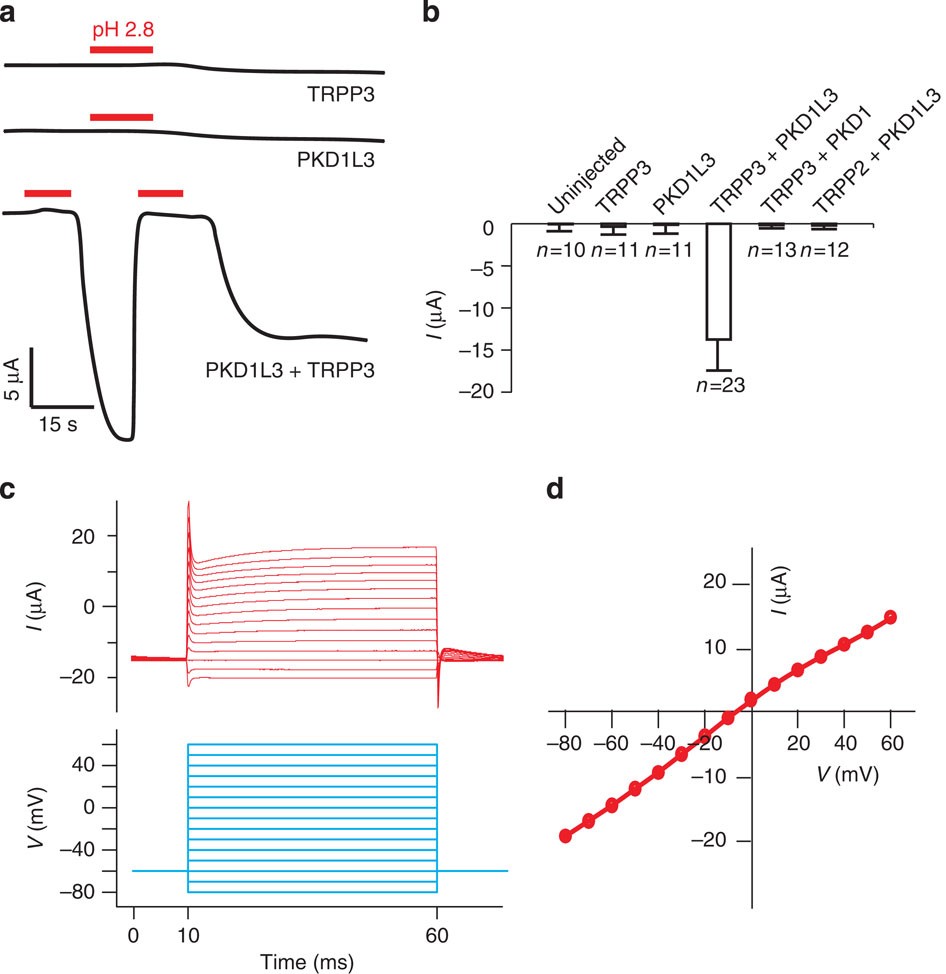
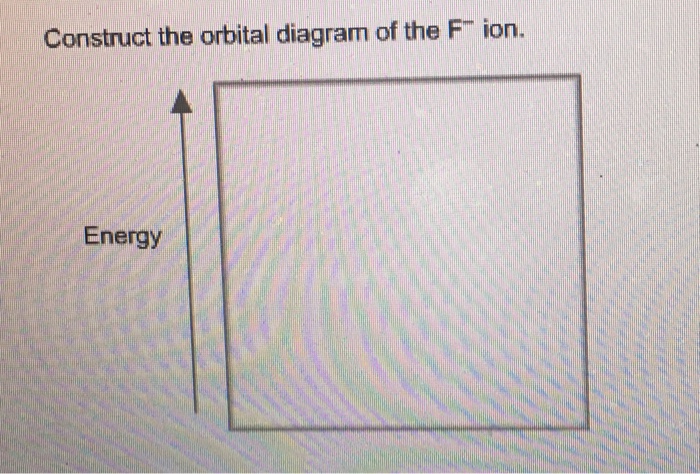
38 construct the orbital diagram of the f– ion.
The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is. Answer to: Construct the orbital diagram of the F^- ion. By signing up, you'll get thousands of step-by-step solutions to your homework questions....1 answer · Top answer: Orbital diagram of F−F− The atomic number of F is 9. After removing one electron, fluorine becomes F−F− and in this case...
Create the atomic orbital diagram for nitrogen. In this case titanium ti is located in period 4 group 4 of the periodic table and has an atomic number of Home study science chemistry chemistry questions and answers construct the orbital diagram of the f ion. How do you draw orbital diagrams.
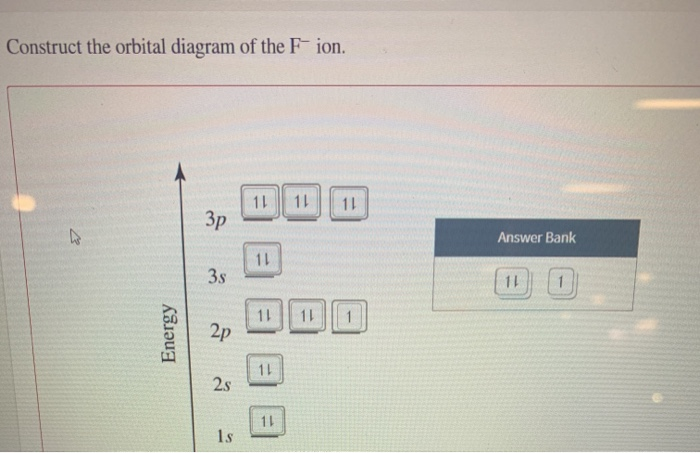
Construct the orbital diagram of the f– ion.
Construct The Orbital Diagram For F Sapling Schematic Diagram. Electron Configuration Wikipedia. Orbital Filling Diagram For Sulfur Great Aluminum Electron. Fluoride Lewis Dot Structure Awesome Bbc Gcse Bitesize Science. Coordination Compounds And Inorganic Reaction Mechanisms Applied. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. Build the orbital diagram for the ion most likely formed by phosphorus? the lium ion has lost an electron, losng this electron from the outermost orbital totally depopulates this orbital, if the orbital has no electrons in it, it doesnt play a part at all in the determination of the size of the atom/ion the lithium...
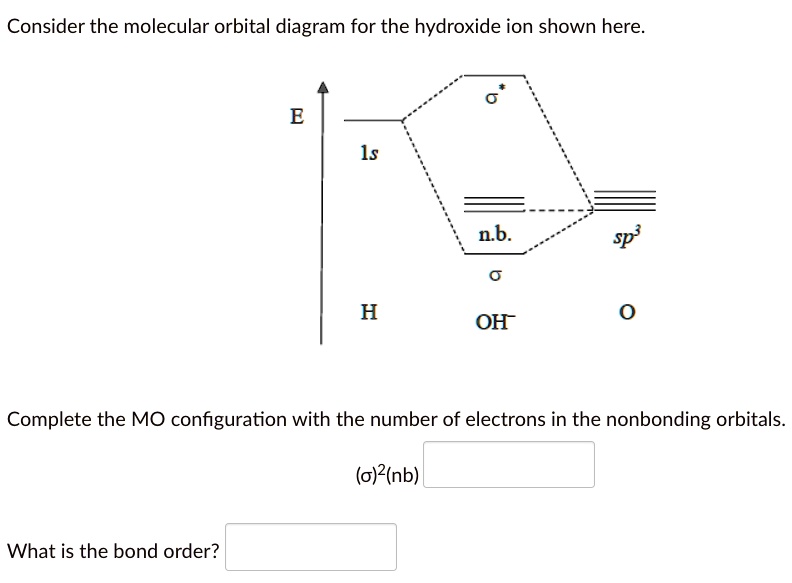
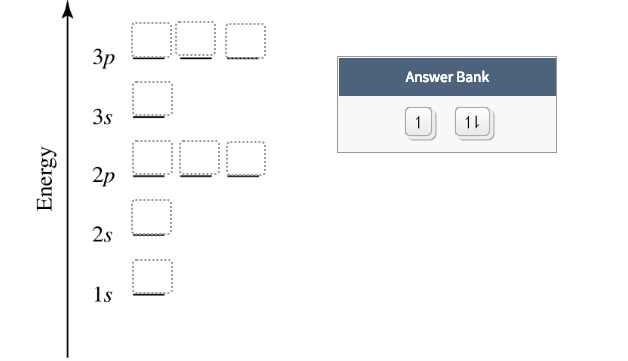
Construct the orbital diagram of the f– ion.. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. Orbital Diagrams & Electron Configurations for Atoms and Ions Orbital diagrams represent the arrangement of electrons in orbitals. • boxes or 2s 2p 2. Orbitals of the same energy level must be filled before moving onto the next energy level. 3. Place electrons singly into orbitals within a sublevel... We will now construct the ground-state electron configuration and orbital diagram for a selection of Orbital diagrams are pictorial representations of the electron configuration, showing the individual Electron Configurations of Ions. We have seen that ions are formed when atoms gain or lose electrons. Transcribed Image Text. Construct the orbital diagram of the F¯ ion. ODD Зр Answer Bank 3s 11 1 ODD 2p 2s 1s Energy .
Electron Box diagrams of the outer electron arrangement and examples of the simple electron notation (e.g The pairs up/down arrows represent a full orbital with electrons of opposite spin and note how the halfñfilled The electron spin box diagrams can be used to show the full electronic structure e.g. The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should... ... orbital isosurface plots in Fig. 3, 5 and 8 were prepared with Molekel 57 and use an isosurface value of AE0.025 e bohr À3 . With the B3LYP hybrid 3. For earlier computational investigations of the electronic structure of Fe(CO) 5 , see, e.g., ref. 58-61. In the D 3h point group the d-orbitals of the iron... The orbital names s, p, d, and f describe electron configuration. These line groups are called sharp, principal, diffuse, and fundamental. s ORBITALS. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre.
Q: 1. Draw the orbital diagram of cobalt ion in Co 3 P 2. 2.Orbital Diagram of N in NH 2 - . Q: According to Lewis dot theory, what types of electron pairs would you find surrounding the central atom of the sulfate i. The electron configuration or orbital diagram of an atom of an element can be deduced from its Then write the orbital diagram, recalling the number of orbitals per sublevel, putting two electrons of Reality Check To construct an orbital diagram, start with the electron configuration and apply Hund... A neutral fluorine atom has 9 electrons. How many electrons does a F^- ion have? Jul 22 2018 06:51 AM. Okay, so we're gonna draw some orbital diagrams. The first one is for lithium. So you want to start by writing its electron configuration but kind of spread out ...5 answers · 8 votes: Draw the following orbitals: a. 3s orbital b. 4s orbital c. 3p orbital
Making positive ions from the d-block ions. This is probably the most unsatisfactory thing about this approach to the electronic structures of the d-block The 4s electrons are also clearly the outermost electrons, and so will define the radius of the atom. The lower energy 3d orbitals are inside them, and...
Orbital Diagrams - Karen Timberlake Orbital Diagrams Orbital Diagram for A Nitrogen Atom N 1s 2s 2p 3s Orbital Diagram for A Fluorine Atom F 1s 2s We can use molecular orbital The orbital energy diagram we obtain is as follows: Grossman, CHE 230 1.13 H Consider the cyclopropenium ion
Be able to construct molecular orbital diagrams for homonuclear diatomic, heteronuclear diatomic, homonuclear Asymmetric diatomic molecules and ions such as CO, NO, and NO+ also Why don't we get sp-orbital mixing for O2 and F2? The reason has to do with the energies of the orbitals, which...
We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Electron Configurations of Ions. Ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more...
According to above diagram structure, configuration energy levels with electron constructed the According to Hund's rule, electrons are filling in the orbital with maximum spin multiplicity. The elements in which the p-orbital are progressively filled by electrons are called p-block in the periodic...
Okay, so we're gonna draw some orbital diagrams. The first one is for lithium. So you want to start by writing its electron configuration but kind of spread out of it. These elements form the basic building blocks of the major macromolecules of life, including carbohydrates, lipids, nucleic acids and proteins.
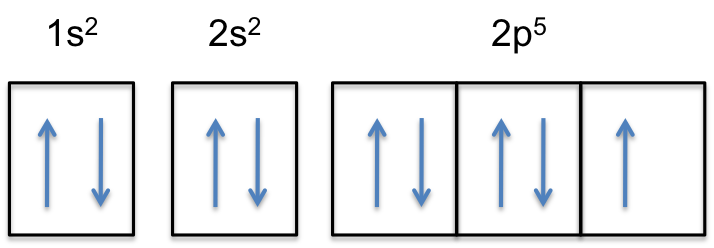
In order to make the ionic compound between two given elements, one should first decide to select which element an an anion (containing negative charge) and Formation of F⁻ Ion: The atomic number of Fluorine is 9. Hence in neutral state it will carry 9 electrons with following electronic configuration
The orbital splitting between the two sets of orbitals (t2g and eg) is designated as the orbital ligand field parameter, δo(where o stands for octahedral). The tendency for complexes to form between a metal ion and a particular combination of ligands and the properties of the resulting complexes...
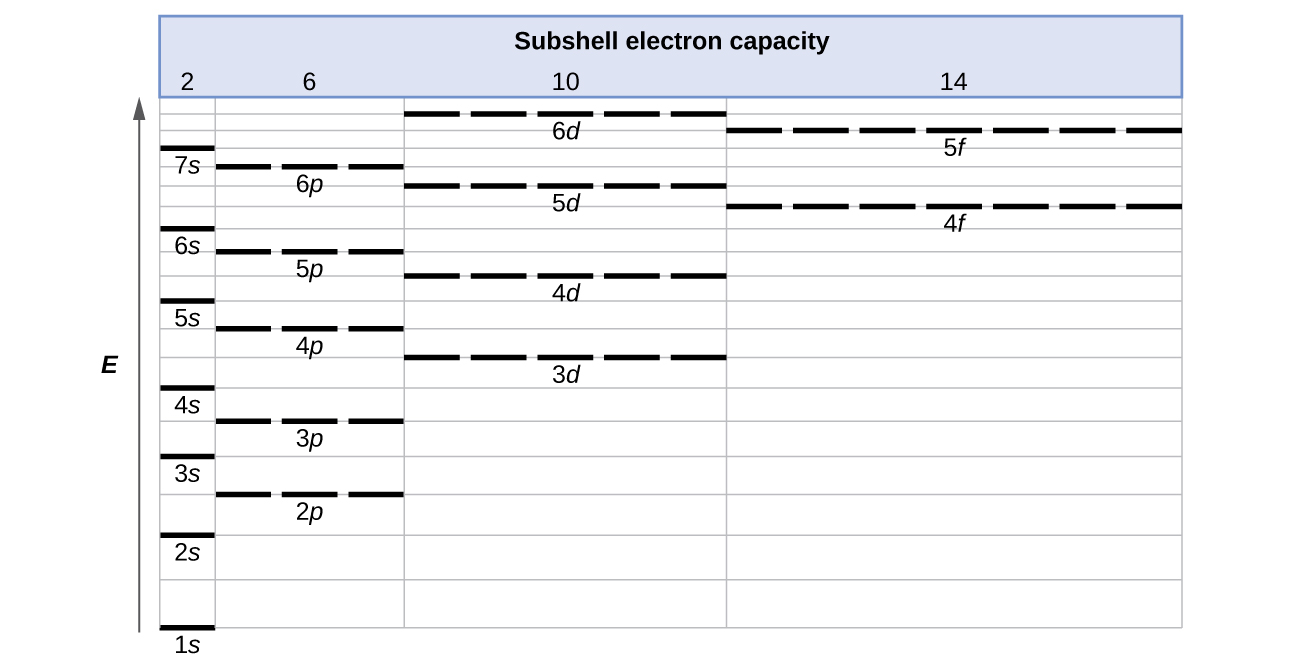
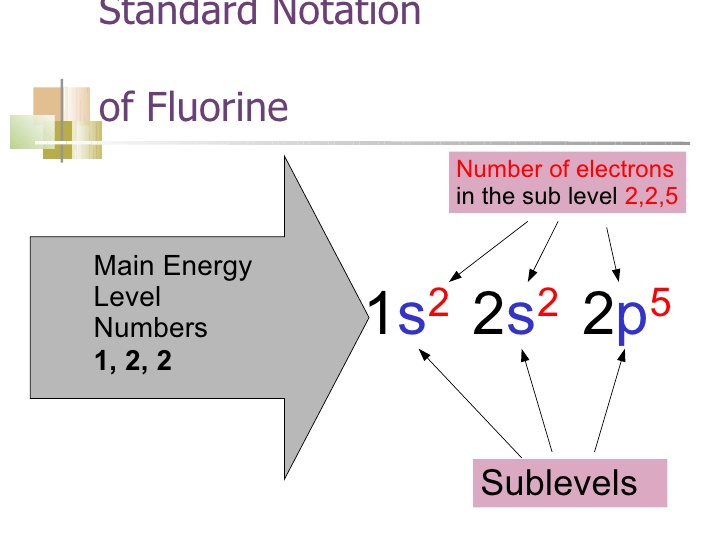
An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. F orbitals make up 7 boxes, and contain a max of 14 electrons (2 in each box). Here is a cheat sheet to remember the order of the orbital diagrams!
S P D F orbitals Explained - 4 Quantum Numbers, Electron Configuration, & Orbital Diagrams.
I'm trying to build a molecular orbital diagram for BF3 and I'm running into problems with irreducible representations on the F side. 2s for B has an With all the formalities out of the way, we can construct the qualitative MO diagram for $\ce{BF3}$. Like much of this post, my source for the MO...
Only RUB 193.34/month. Electron Configurations and Orbital Diagrams. Terms in this set (18). C (carbon). What element does the following orbital diagram represent? Mg (magnesium).
True or False 1.The contour of the orbital would extend further out along the x and y axes. Which statement is true about the motion of a planet in its orbit around the sun? A) the orbital speed increases as the planet approaches apogee B) the planets orbital speed changes as it moves closer...
Transcribed image text: Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons. How many electrons does a F^- ion have?
Build the orbital diagram for the ion most likely formed by phosphorus? the lium ion has lost an electron, losng this electron from the outermost orbital totally depopulates this orbital, if the orbital has no electrons in it, it doesnt play a part at all in the determination of the size of the atom/ion the lithium...
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital.
Construct The Orbital Diagram For F Sapling Schematic Diagram. Electron Configuration Wikipedia. Orbital Filling Diagram For Sulfur Great Aluminum Electron. Fluoride Lewis Dot Structure Awesome Bbc Gcse Bitesize Science. Coordination Compounds And Inorganic Reaction Mechanisms Applied.



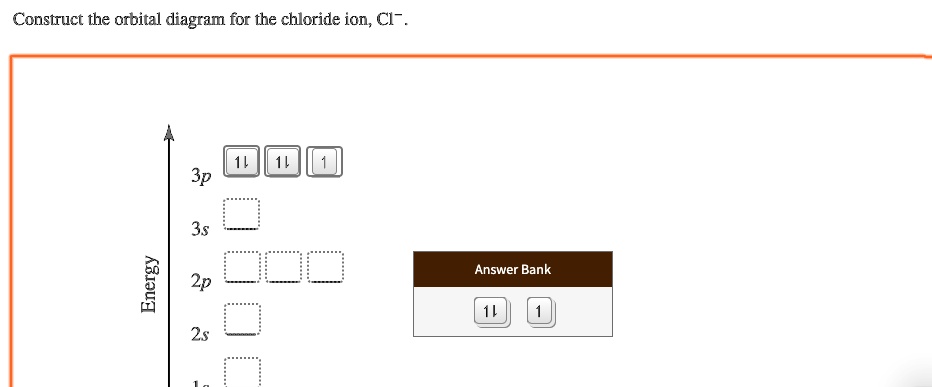





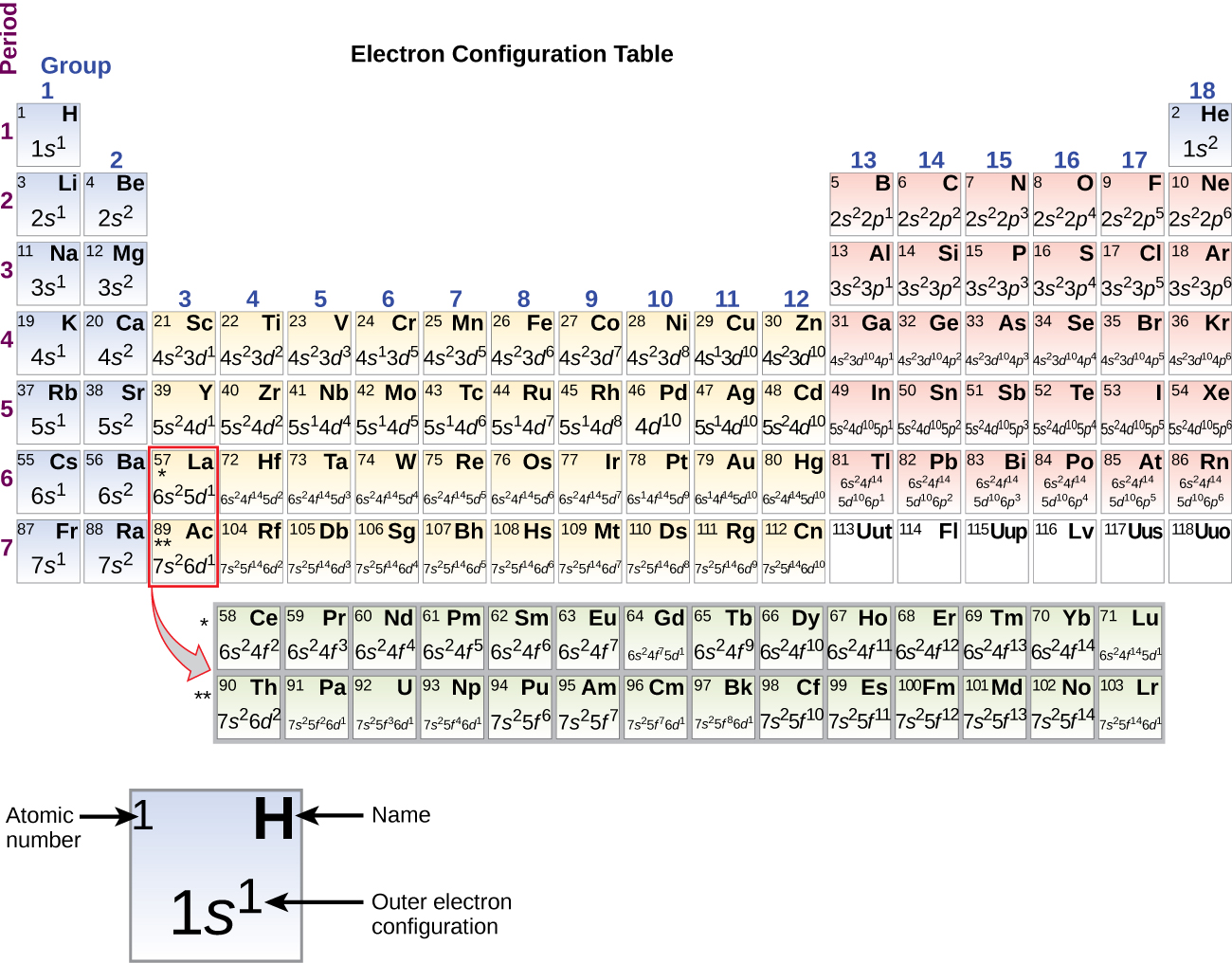
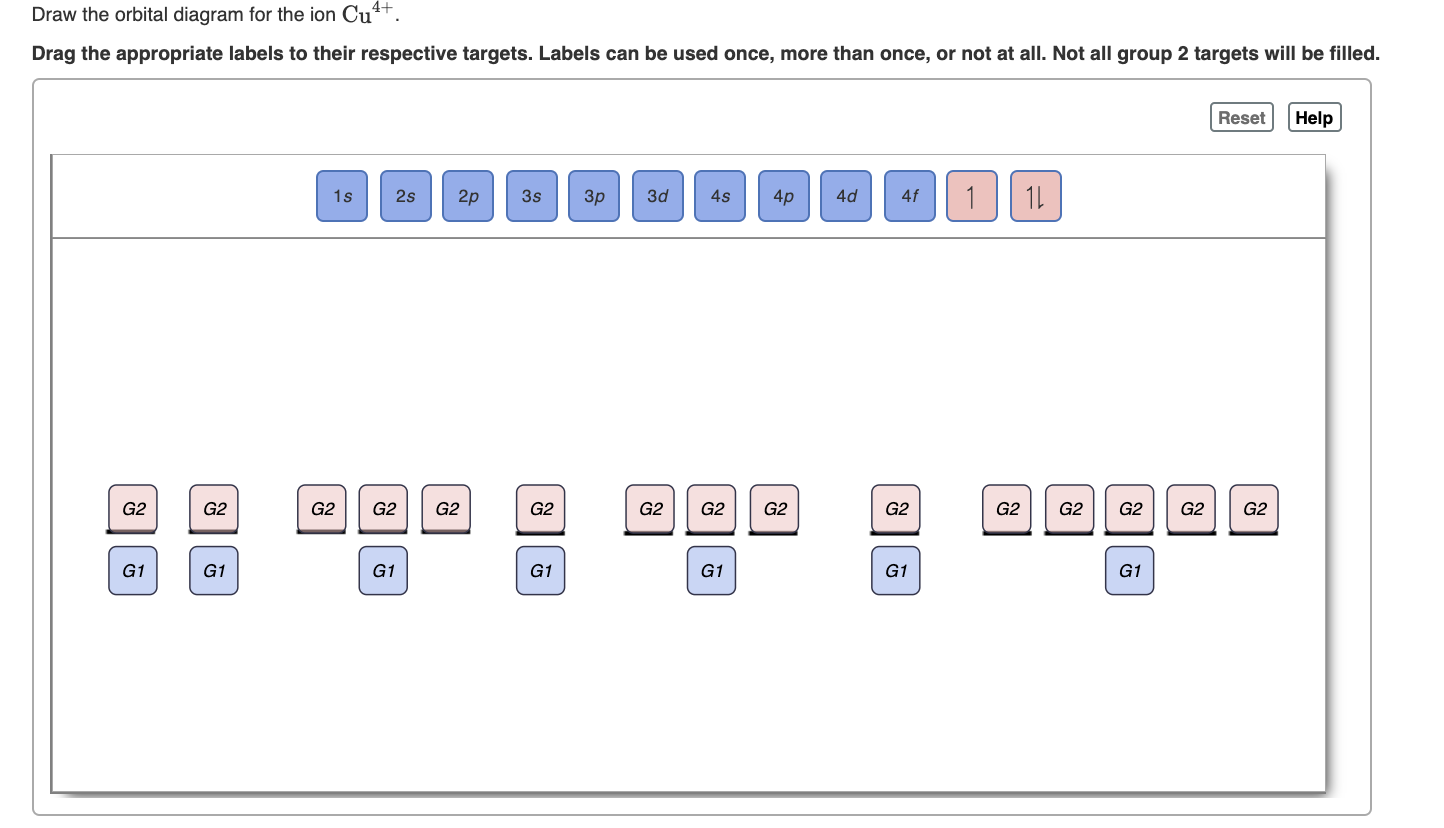

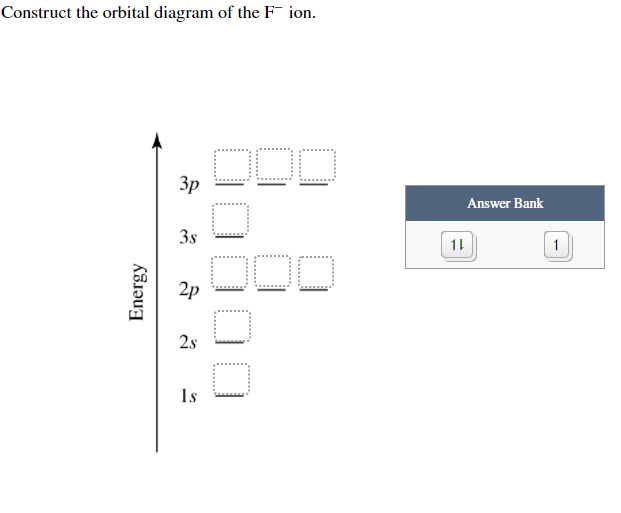










0 Response to "38 construct the orbital diagram of the f– ion."
Post a Comment