38 hf molecular orbital diagram
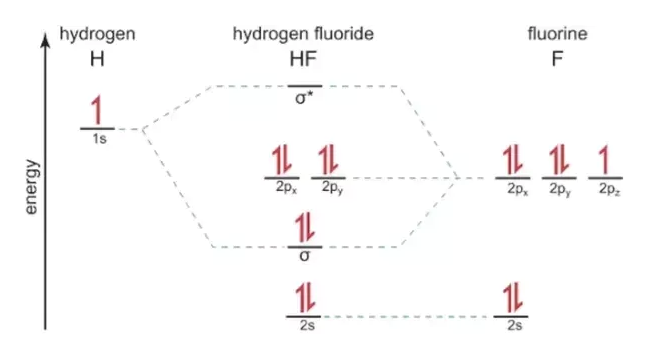
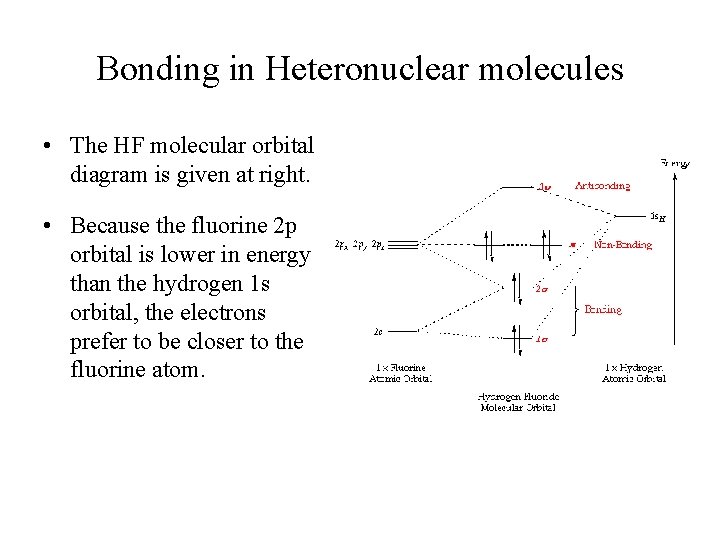
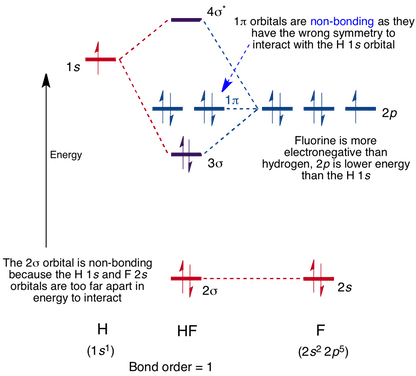
The molecular orbital diagram of HF looks different than most other diatomic species because the electronegativity difference between H and F is so large. The 1s atomic orbital of H interacts with just one of the 2p atomic orbitals of F to form a bonding o molecular orbital and an antibonding o molecular orbital. Hydrogen fluoride is an ionic compound that is formed by hydrogen and the most electronegative atom fluorine. Hydrogen and fluorine are bonded together by an ...1 answer · Top answer: It is a diatomic molecule that contains two different atoms in which one is more electronegative. And the one which is more electronegative will have...
The molecular orbital diagram of HF looks different than most other diatomic species because the electronegativity difference between H and F is so large. The 1s atomic orbital of H interacts with just one of the 2p atomic orbitals of F to form a bonding o molecular orbital and an antibonding o* molecular orbital.
Hf molecular orbital diagram
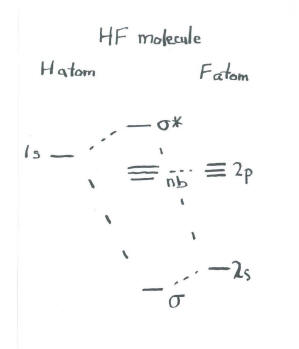
An Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2000 1 Introduction Hartree-Fock theory is fundamental to much of electronic structure theory. It is the basis of molecular orbital (MO) theory, which posits that each electron's motion can be ... Download scientific diagram | Molecular orbital diagrams for HBr and HF. from publication: Total energy partitioning within a one-electron formalism: A Hamilton population study of surface-CO ... Molecular Orbital Diagram for the HF Molecule. Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the formation of a sigma-bonding and a sigma-antibonding molecular orbital, as shown below. Figure 1: Molecular orbitals of HF. (CC BY-SA-NC 2.0 UK: England & Wales License; Nick Greeves).
Hf molecular orbital diagram. Molecular orbitals of HF. Draw the MO diagram for HBr including energy levels each orbitals shape each orbitals character meaning what atomic orbitals contribute to each MO. H1 is bonded in a linear geometry to two equivalent Br1- atoms. And how much symmetry labels eg. And how much symmetry labels eg. basic idea behind molecular orbital theory - there are many variations on the bystep and deal with H2 and then Li2 and then LiH we will instead begin by. Molecular orbital energy level diagram for homonuclear diatomic molecules .. Density diagrams of the molecular orbitals for the LiH, CH, and HF molecules are .Molecular orbital. much of each atomic coefficient is required to make each of the molecular orbitals. The coefficients for the highest energy MO wavefunction is solved below. Repeat these calculations for determining the coefficients of this molecular orbital, but then go on to calculate the coefficients for the other two MO's in our HF molecule. Which is the molecular orbital diagram for HF? The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already explained ,only a pz orbital is able to combine with s orbital.
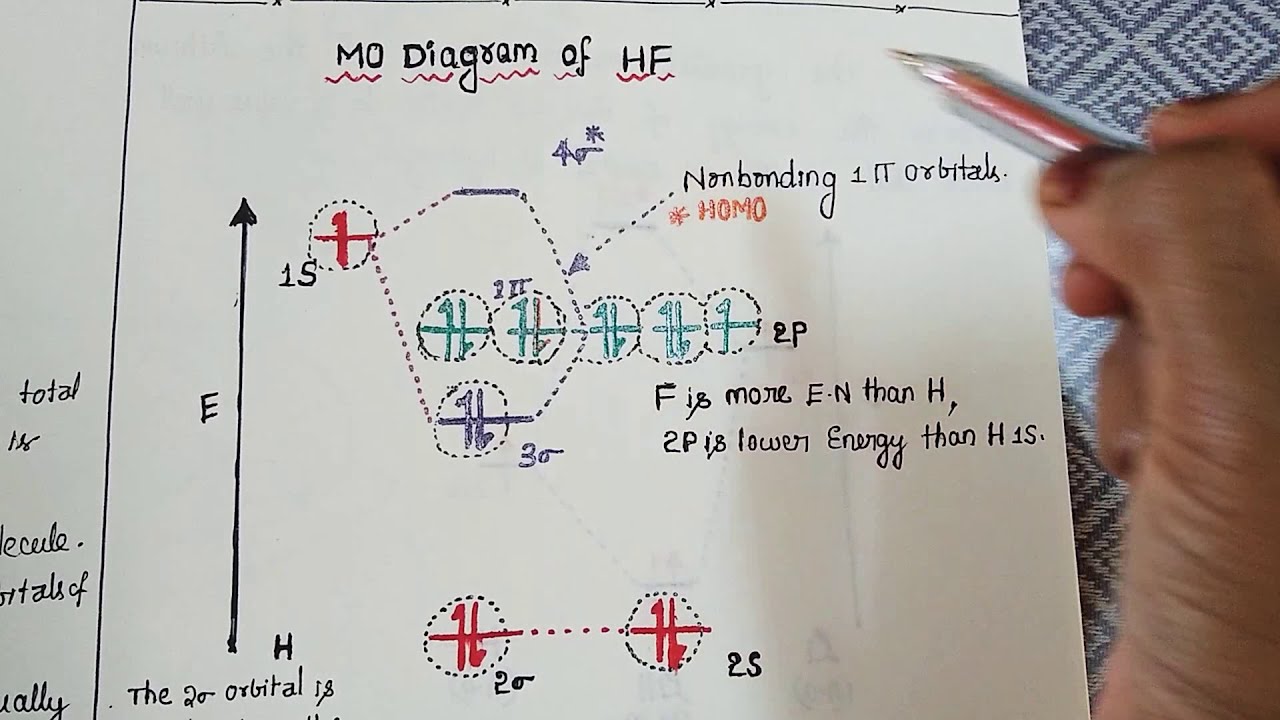
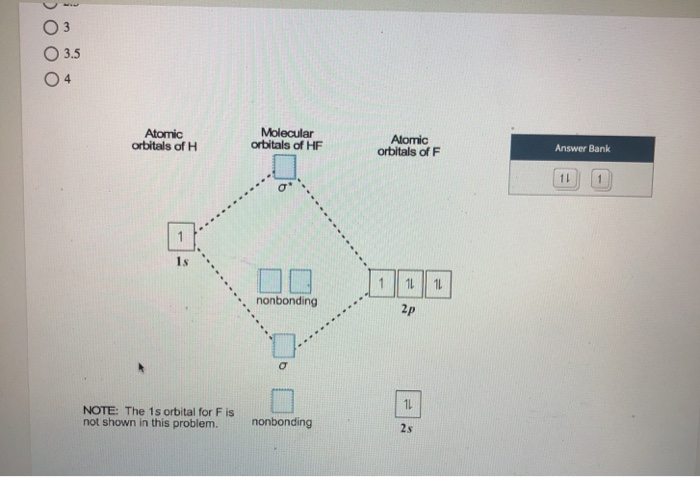
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in The molecular orbitals which involve ps orbitals are characteristically strongly polarized in a direction away from the bond in the region of the nucleus on which the p orbital is centred. Compare, for example, the 3 s orbitals of CH and HF with the 3 s g molecular orbital of the homonuclear diatomic molecules. Hartree-Fock Molecular Orbital Theory 1. Invoke the Born-Oppenheimer approximation 2. Express the electronic wavefunction as a single Slater Determinant 3. Solve for those orbitals which minimize the electronic energy (variational method) This winds up being mathematically equivalent to assuming each electron interacts only with the average Drawn below is an incomplete molecular orbital (MO) diagram for the molecule HF. ... A nonbonding electron is promoted to an anti bonding orbital.3 pages
Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Hydrogen Fluoride. Molecular orbitals in Hydrogen Fluoride. CONTROLS . Click on the HF molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. HF HOMO Orbital The HOMO orbital is the highest energy molecular orbital occupied by electrons. In HF, the HOMO orbitals are the double degenerate pi 2px and 2py and pi orbitals. To get a 3-D model you can manipulate, click here. Download time may be significant the first time the applet is loaded. HF LUMO Orbital Answer (1 of 2): Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl have comparable energy with the ... Tutorial: Display of Orbitals and Molecular Surfaces. 3. Natural Molecular Orbital Analysis. The occupied canonical orbitals that are obtained from a Hartree-Fock (HF) calculation are generally delocalized. In many situations, this is undesirable because it is difficult to attach a chemical interpretation to these molecular orbitals.
Answer (1 of 3): The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already explained ,only a pz orbital is able to combi...
Construct the molecular orbital diagram for HF. Use only the valence electrons for your diagram. The 2s orbital of F atom has an energy more than 26 eV lower than that of the H 1s, so there is very little interaction between them. The F 2p orbital (-18.65 eV) and the H 1s (-13.61 eV), on the other hand, have similar energies, allowing them to
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
valence orbitals. A first-row atom - the most useful polarization functions are d GTOs, hydrogen - p GTOs. Adding d functions to the nitrogen basis set causes HF theory to predict correctly a pyramidal minimum for ammonia. A variety of other molecular properties are sensitive to the presence of polarization
In this screencast, Andrew Burrows walks you through how to construct the MO energy level diagram of HF. http://ukcatalogue.oup.com/product/9780199691852.do#...
#MOT #BMO #ABMO #HF #CO #NO #CN #OHHello everyoneThis is shivam here To follow me on instagram search - Sshivam898To join telegram group click on the given l...
This discussion on Molecular orbital diagram of HF2-? is done on EduRev Study Group by Chemistry Students. The Questions and Answers of Molecular orbital diagram of HF2-? are solved by group of students and teacher of Chemistry, which is also the largest student community of Chemistry.
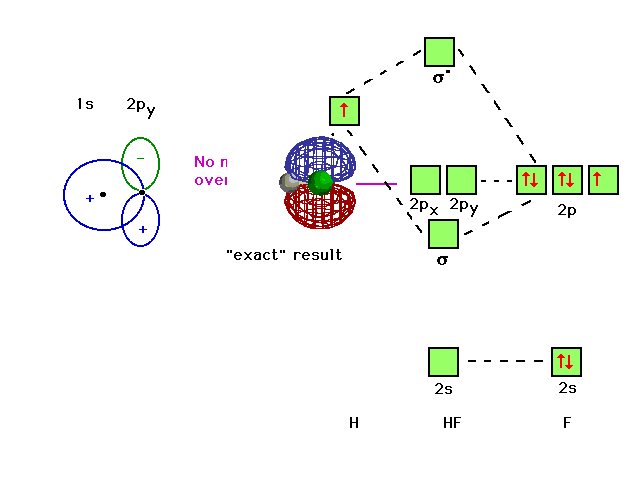
Also, 1 π electrons are completely localised on the F atom because the 2 p x and 2 p y orbitals on F have a zero net overlap with the 1 s orbital on H. Electrons in MOs localized on a single atom are referred to as nonbonding electrons. Also, I would note that the 3 σ MO has less bonding character and the 4 σ ∗ MO has less anti-bonding ...
21 Oct 2016 — In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already ...3 answers · 37 votes: The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation ...What is the molecular orbital diagram for HCL?2 answers20 Aug 2015What is the molecular orbital diagram of O2 and F2?6 answers12 Mar 2017What is the molecular orbital energy diagram of CO?4 answers7 Jan 2017What is the molecular orbital diagram of HCl and how?1 answer11 Dec 2017More results from www.quora.com
Illustrating molecular orbitals (valence electrons only) in HF whose atoms are very different in electronegativity. The lowest molecular orbital is virtually indistinguishable from the 2s atomic orbital on F (because of the large energy difference with H's atomic orbital).
Example 2: hydrogen fluoride When atoms are of different energies, one must be concerned with the relative energies and symmetries of orbitals Orbitals of same symmetry and approximately similar energy combine most effectively Can estimate approximate HF molecular orbitals Energies calculated with Gaussian Gives filling order of orbitals
Molecular Orbital Diagram for the HF Molecule. Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the formation of a sigma-bonding and a sigma-antibonding molecular orbital, as shown below. Figure 1: Molecular orbitals of HF. (CC BY-SA-NC 2.0 UK: England & Wales License; Nick Greeves).
Download scientific diagram | Molecular orbital diagrams for HBr and HF. from publication: Total energy partitioning within a one-electron formalism: A Hamilton population study of surface-CO ...
An Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2000 1 Introduction Hartree-Fock theory is fundamental to much of electronic structure theory. It is the basis of molecular orbital (MO) theory, which posits that each electron's motion can be ...


















0 Response to "38 hf molecular orbital diagram"
Post a Comment