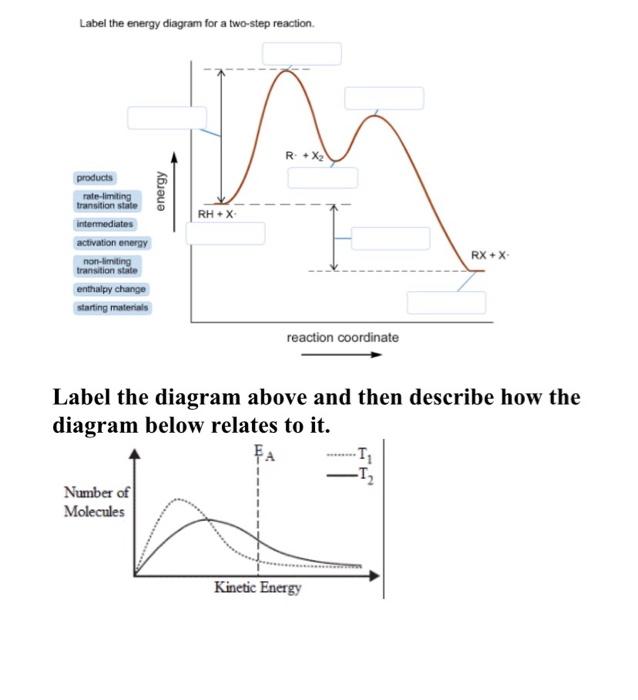
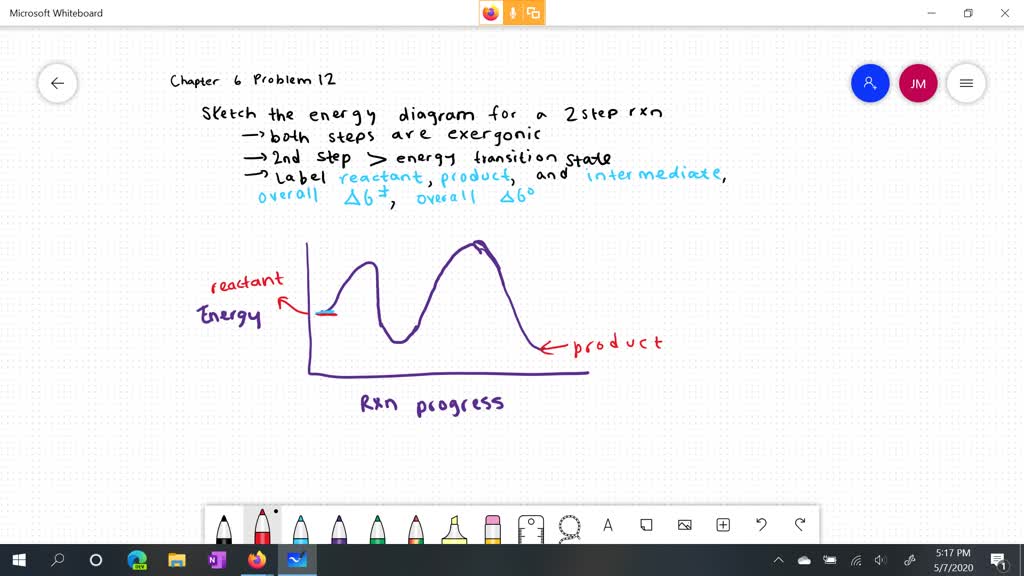
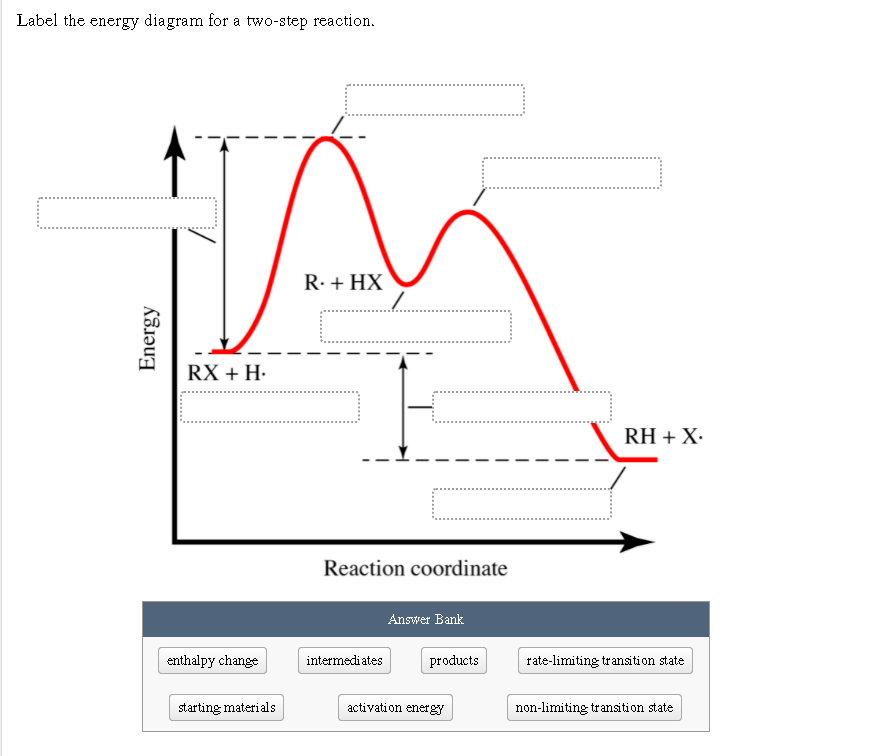
38 label the energy diagram for a two-step reaction
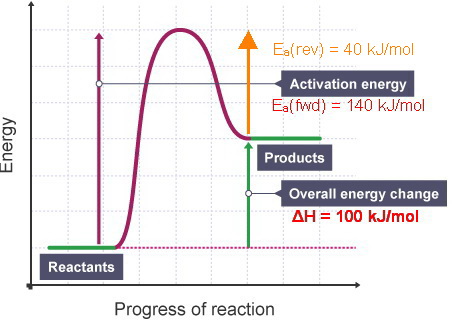
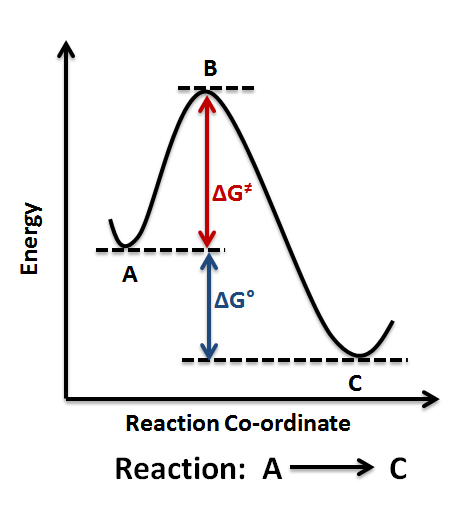
Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. We’re being asked to label the given energy diagram. Recall that an energy diagram is usually read from left to right. The components of a two-step energy diagram are: • Reactants: are placed on the left/beginning of the energy diagram • Products: are placed on the right/end of the energy diagram • Non-limiting transition state: is the transition state with the lowest energy in the energy diagram
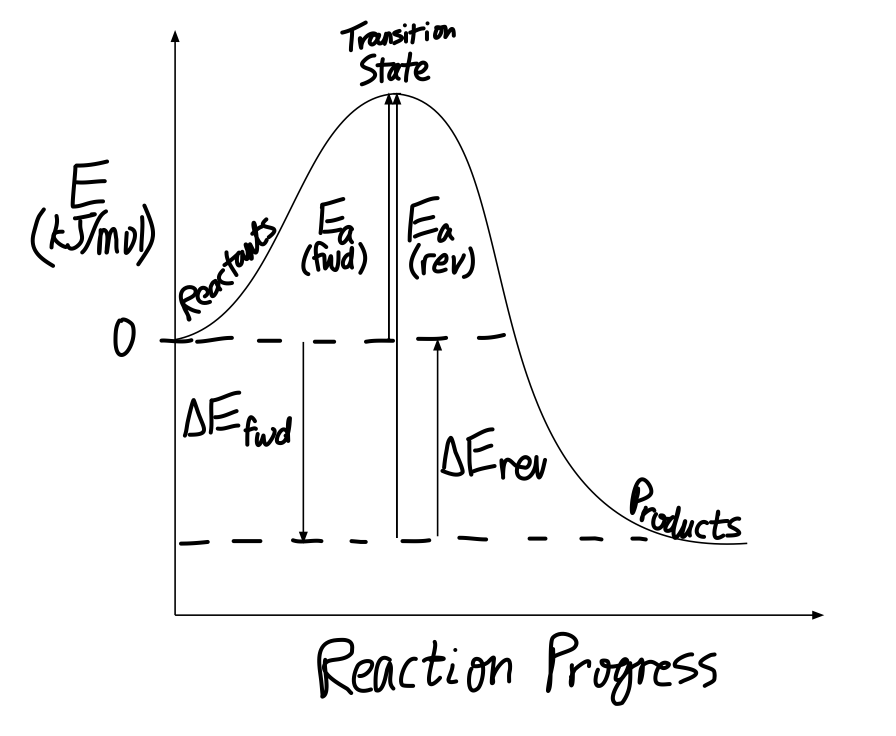
57CP. Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, ΔH°, and E a. a. A concerted, exothermic reaction with a low energy of activation. b. A one-step endothermic reaction with a high energy of activation. c. A two-step reaction.
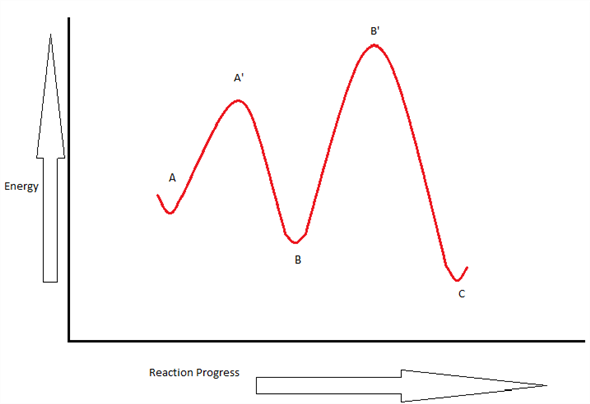
Label the energy diagram for a two-step reaction
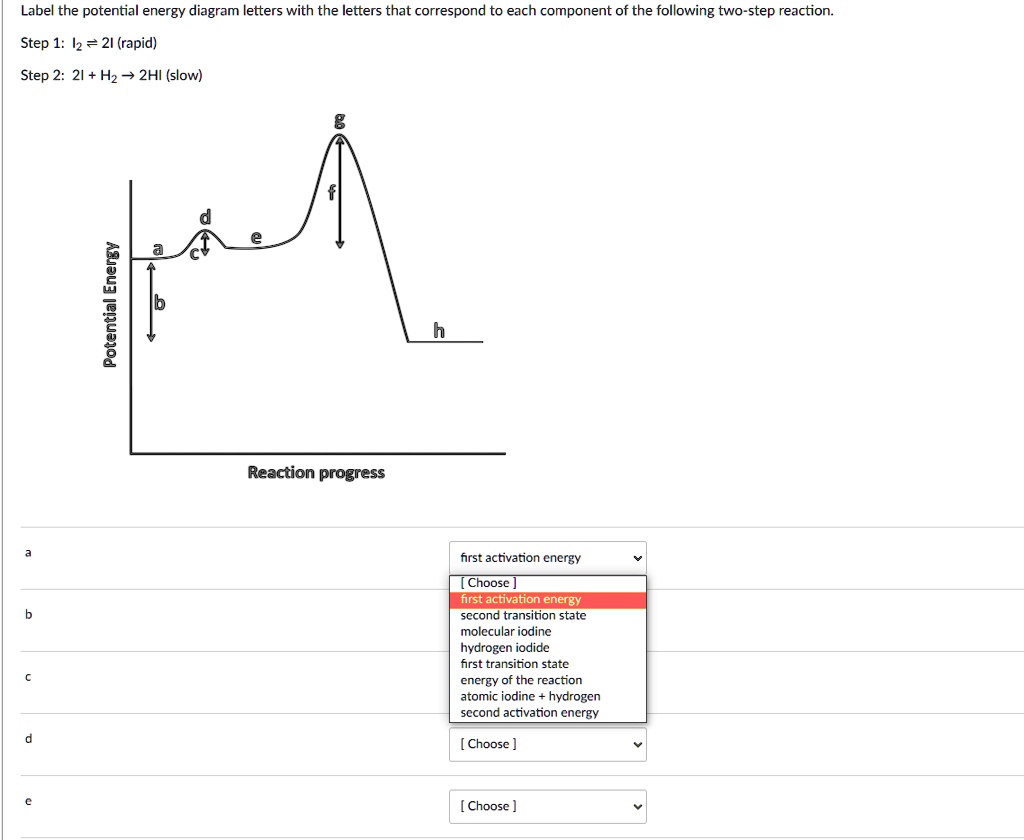
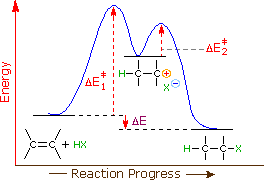
is the complex created in the first reaction, while is the activated complex created in the second reaction. Thus, for this two-step process, there are two activated complexes. Example: Draw the potential energy diagram for the following multi-step reaction . Properly label the diagram. Solution: Rate of Reaction is Determined by Slowest Step Draw and label potential energy diagram for the reaction including a molecular structure that could represent an activated complex. The activated complex would show an unstable association of one CH 4(g) molecule and O 2(g) molecule with partial bonds. Check Your Solution The potential energy diagram should match the given information. Organic Chemistry (9th Edition) Edit edition Solutions for Chapter 6 Problem 21E: Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall ΔG°, transition states, and intermediate.
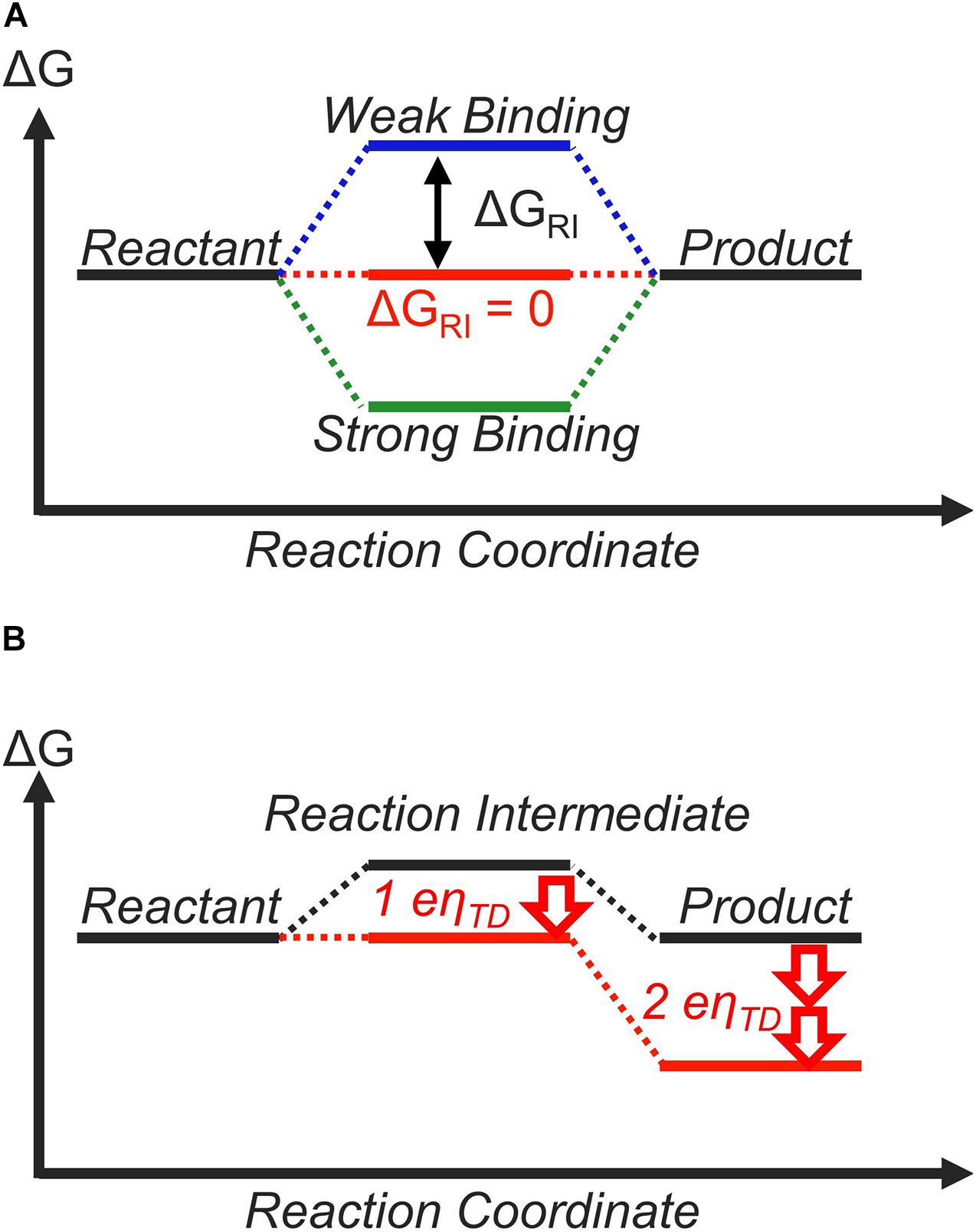
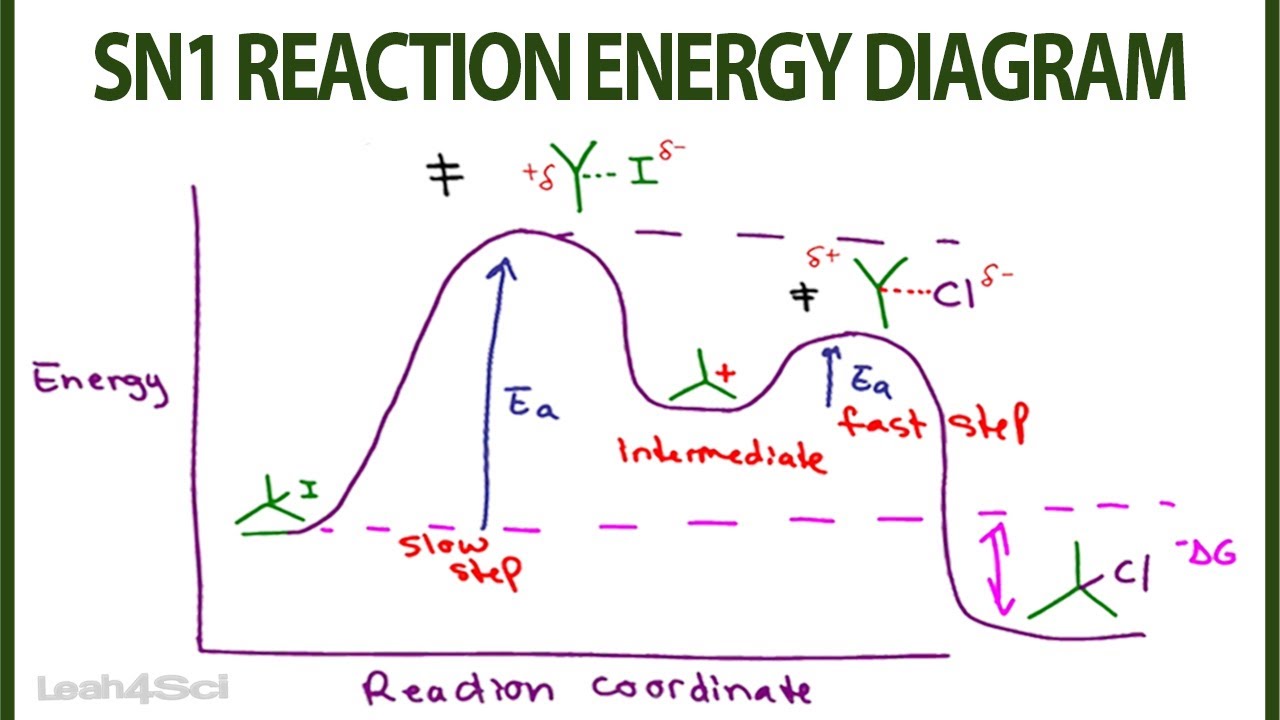
Label the energy diagram for a two-step reaction. A Two-Step Reaction Mechanism. The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Step 1 has the higher transition energy state, thus it is the rate-determining step. Sn2 is a one step, 2 arrow mechanism with alkyl halides. Sn1 is a two step, 2 arrow mechanism (often followed by a deprotonation step) with alkyl halides. Problem 1: Label each as Sn1 or Sn2. Provide arrow mechanisms for each reaction, and draw an energy diagram assuming an overall exothermic process. The reaction profile of a two-step reaction is represented by a potential energy diagram. The y-axis depicts the potential energy of the reacting species, ... Potential Energy DiagramsPotential Energy Diagrams o Generally, if a reaction requires a low activation energy, it is considered to be a spontaneous reaction (i.e. It is generally a fast reaction) • Reaction Mechanism o Elementary Process • A process in which a reaction occurs in one step (usually occurs between 2 particles) Ternary Process
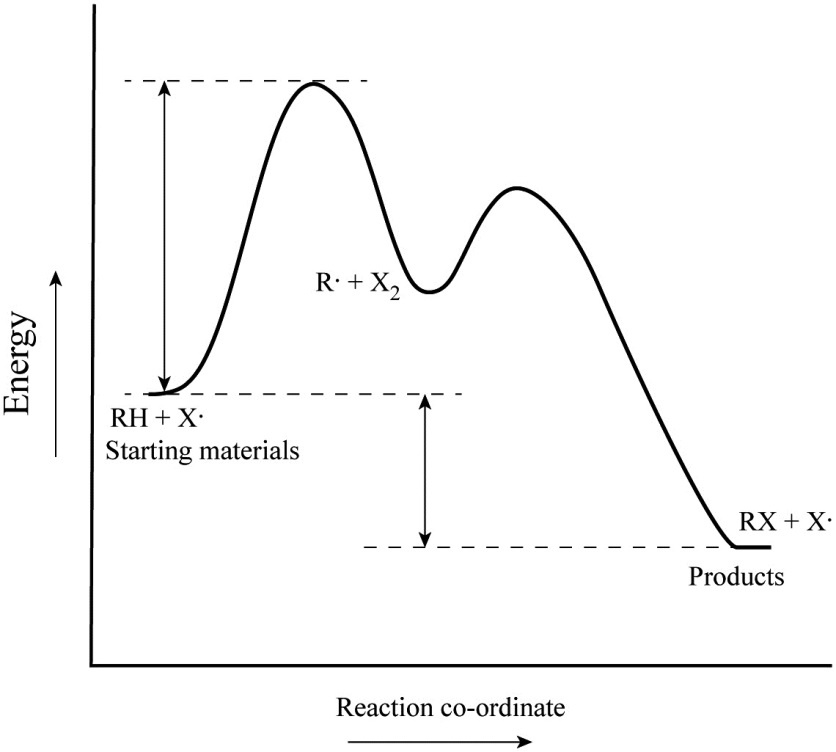
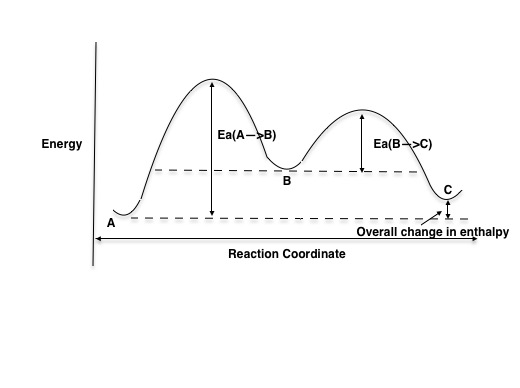
The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product. Transcribed image text: Label the energy diagram for a two-step reaction. R: + HX Energy RX + H RH + X Reaction coordinate Reaction coordinate Answer Bank ... Drag the labels onto the diagram to identify the steps in a reaction both with and without enzymes. Reset Help Activation energy required Stable product Reactants Energy With enzyme 3 Without enzyme Progress of reaction. Question: Drag the labels onto the diagram to identify the steps in a reaction both ... A Two-Step Reaction Mechanism We draw an energy diagram for each step, and then combine them in an energy diagram for the overall two step mechanism. Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram.
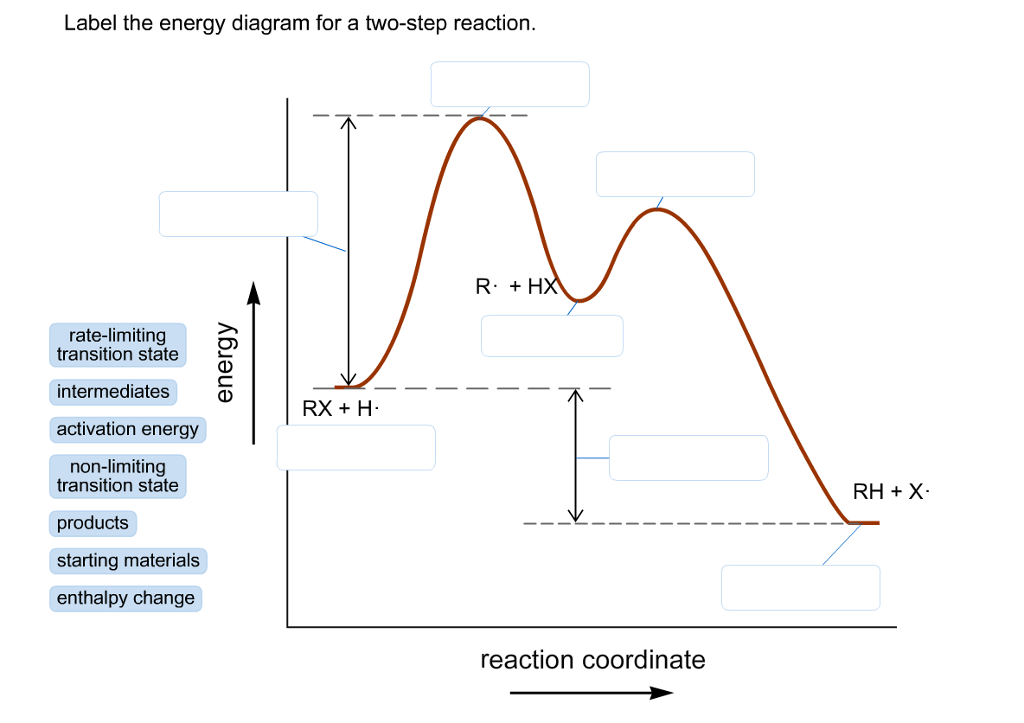
Transcribed image text: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting ... Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows% (1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher ... I mean, it probably wouldn't be as good as regular thermonuclear weapons, since it *has* to be deployed from orbit. But say we lived in a world where all fissionable and fertile substances are gone. Say, for example, on a planet in a star system with low metallicity. Wouldn't this design allow us to still find a way to threaten ourselves with mass extinction? Chemistry questions and answers. Label the energy diagram for a two-step reaction. R. + HX Energy RX + H . RH + X Reaction coordinate Answer Bank enthalpy change intermediates products rate-limiting transition state starting materials activation energy non-limiting transition state.
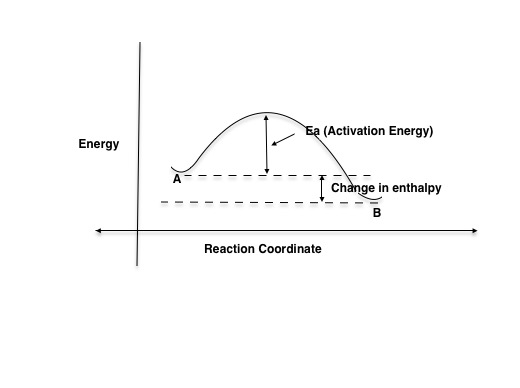
A complete enthalpy diagram will include starting energy, ending energy, and E a and delta H. This enthalpy diagram has starting products, ending products, delta H, and activation energy labeled...
19 Mar 2018 — Label the energy diagram for a two-step reaction. ... An energy profile diagram is a theoretical representation that shows how the energy of the ...
This is an example of a hydrolysis reaction (urea plus water). For each equivalent of carbon dioxide (CO2CO2), two equivalents of ammonia (NH3NH3) are produced. Label the enzyme, substrate, enzyme-substrate complex, enzyme-product complex, and product in the enzymatic reaction for the breakdown of urea by urease using the induced-fit model.
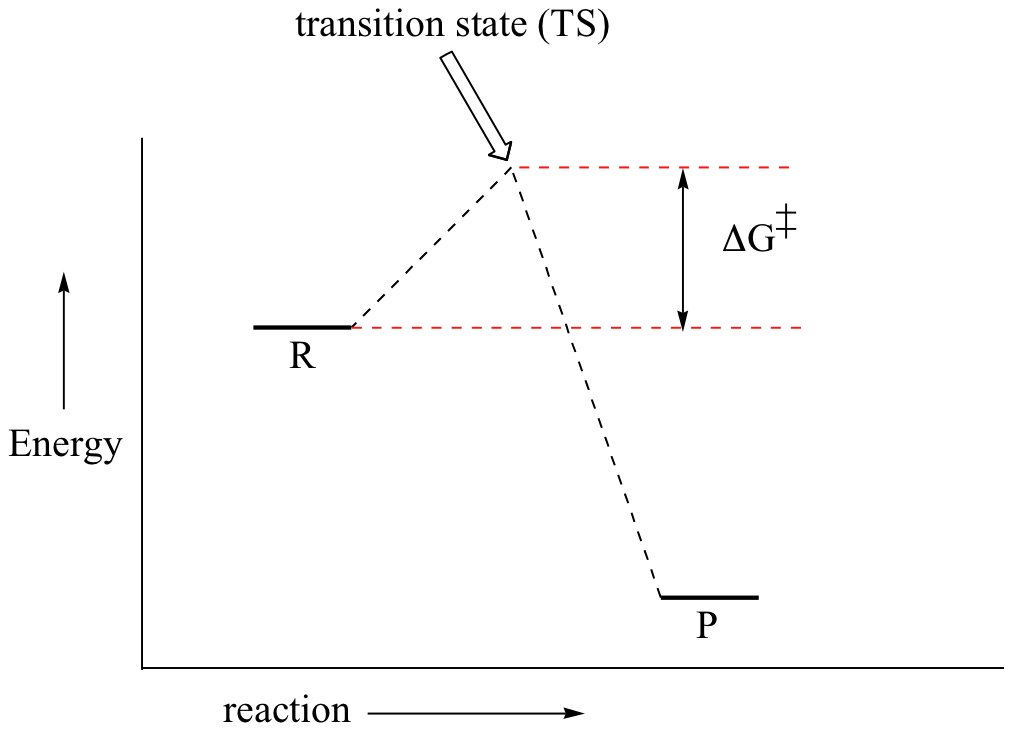
Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall Δ G †, and overall Δ G ∘. Jil M. Numerade Educator 07:16 Problem 14
Draw a potential energy diagram for a two-step endothermic reaction. The first step is the rate determining step. Label all axis and indicate the location of the following: reactants, products, intermediate, activated complex, and transition state.
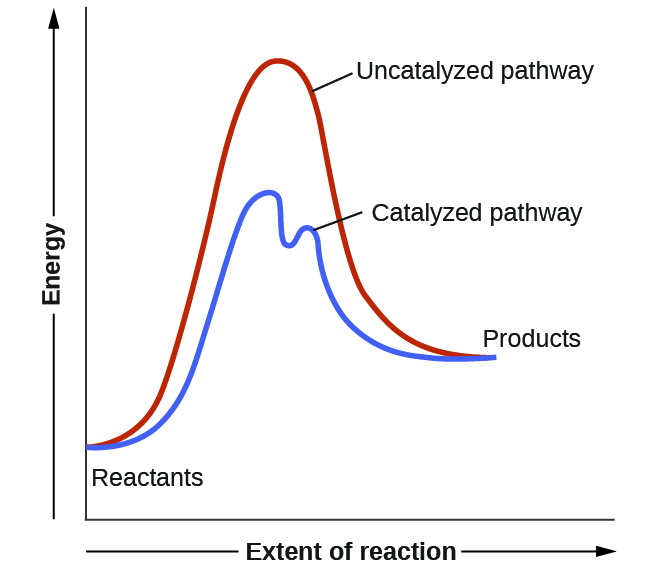
Using Reaction Diagrams to Compare Catalyzed Reactions The two reaction diagrams here represent the same reaction: one without a catalyst and one with a catalyst. Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Solution
18P Draw a reaction energy diagram for a two-step reaction that has an endothermic first step and an exothermic second step. Label the reactants, transition states, reaction intermediate, activation energies, and enthalpy differences. Step-by-step solution 100% (5 ratings) for this solution Chapter 3, Problem 18P is solved. View this answer
While the central bankers' asset bubbles and Ponzi markets hit new highs, the real economy is breaking down as inflation soars. One of these things is not like the other. [https://www.dailymail.co.uk/news/article-10210741/Gas-prices-jump-17-day-consumers-warned-brace-bills-soaring-475-year.html](https://www.dailymail.co.uk/news/article-10210741/Gas-prices-jump-17-day-consumers-warned-brace-bills-soaring-475-year.html) UK gas prices jumped 17 per cent in a day after two more energy firms colla...
Clearly, RX + H. represents the rectants or the starti …. View the full answer. Transcribed image text: Label the energy diagram for a two-step reaction. R. + HX rate-limiting transition state energy intermediates RX + H activation energy non-limiting transition state RH + X: products starting materials enthalpy change reaction coordinate.
The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Solution for Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate,…
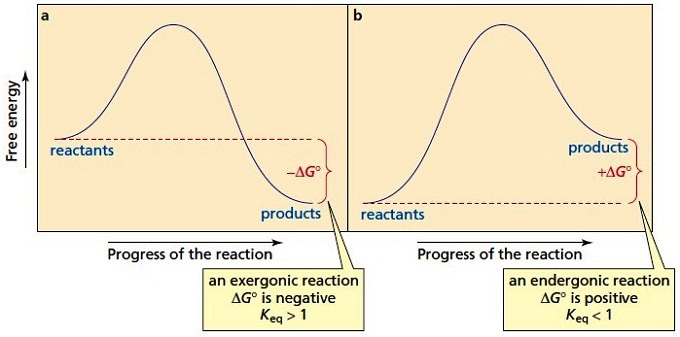
Explanation: The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants.
Problem: Label the energy diagram for a two-step reaction. FREE Expert Solution Show answer Answer: 84% (178 ratings) Sign up for free to keep watching this solution Sign up for free. 581,500. students enrolled ... Label the energy diagram for a two-step reaction.
Consider the one-step conversion of F to G. Given that the reaction is endothermic by 5 kcal/mol and that the energy difference between G and the transition state for the process is 15 kcal/mol, sketch a reaction-energy diagram for this reaction. Make sure to show how the given energy differences are consistent with your sketch.
Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn, as well as DG 1 * and DG 2 * for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. ?
Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states.
See the answer See the answer done loading. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (42 ratings) Transcribed image text: Label the energy diagram for a two-step reaction.
Draw a potential energy diagram for a two-step endothermic reaction. The first step is the rate determining step. Label all axis and indicate the location of the following: reactants, products, intermediate, activated complex, and transition state.
I’m new to this event, can I do this?
Organic Chemistry (9th Edition) Edit edition Solutions for Chapter 6 Problem 21E: Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall ΔG°, transition states, and intermediate.
Draw and label potential energy diagram for the reaction including a molecular structure that could represent an activated complex. The activated complex would show an unstable association of one CH 4(g) molecule and O 2(g) molecule with partial bonds. Check Your Solution The potential energy diagram should match the given information.
is the complex created in the first reaction, while is the activated complex created in the second reaction. Thus, for this two-step process, there are two activated complexes. Example: Draw the potential energy diagram for the following multi-step reaction . Properly label the diagram. Solution: Rate of Reaction is Determined by Slowest Step












0 Response to "38 label the energy diagram for a two-step reaction"
Post a Comment