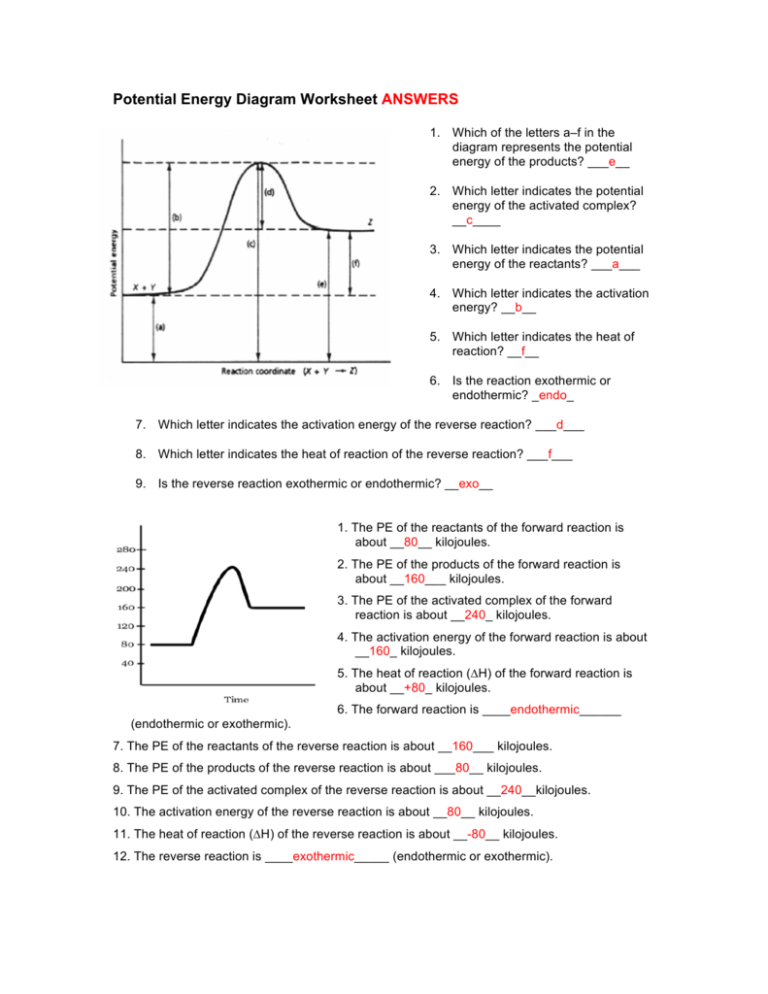
38 Potential Energy Diagram Endothermic
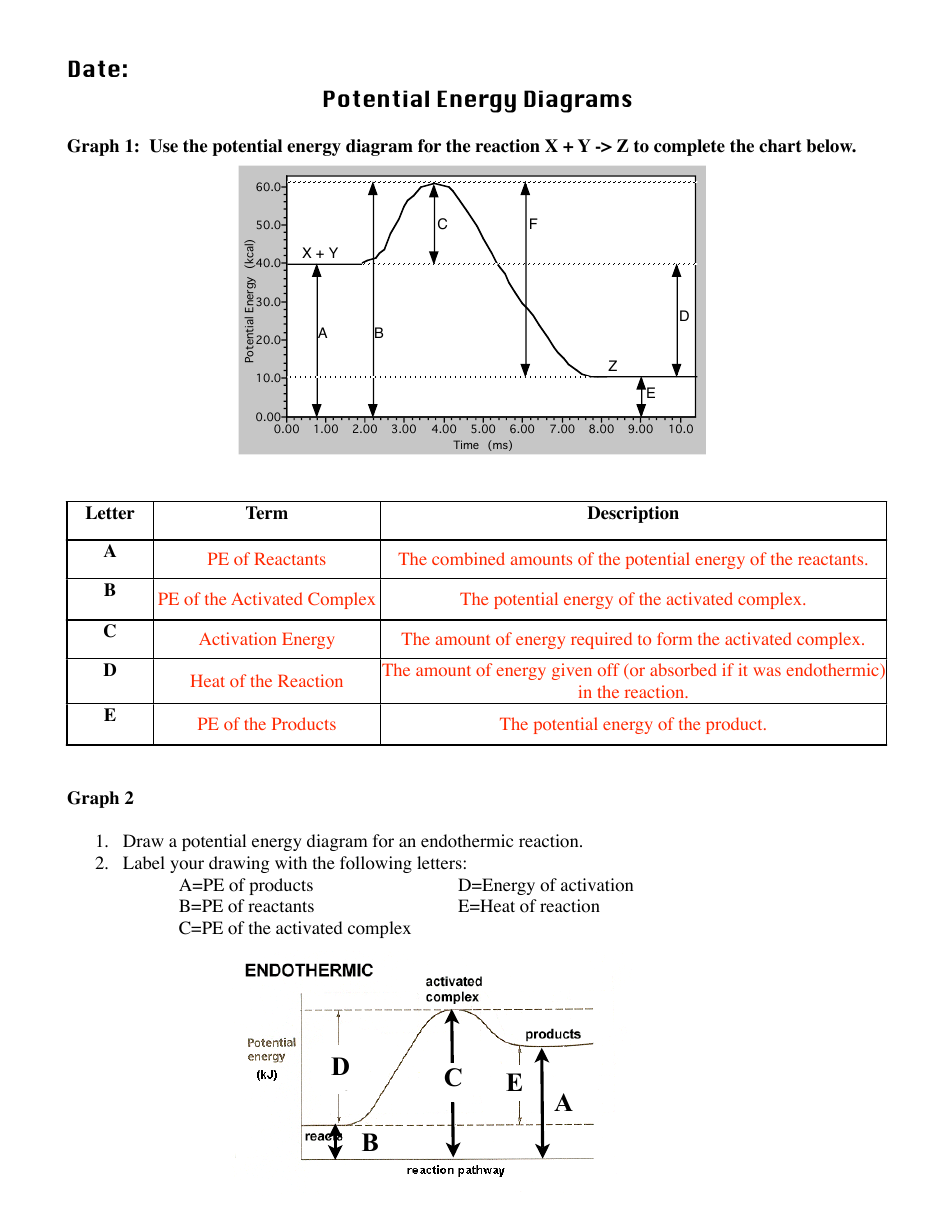
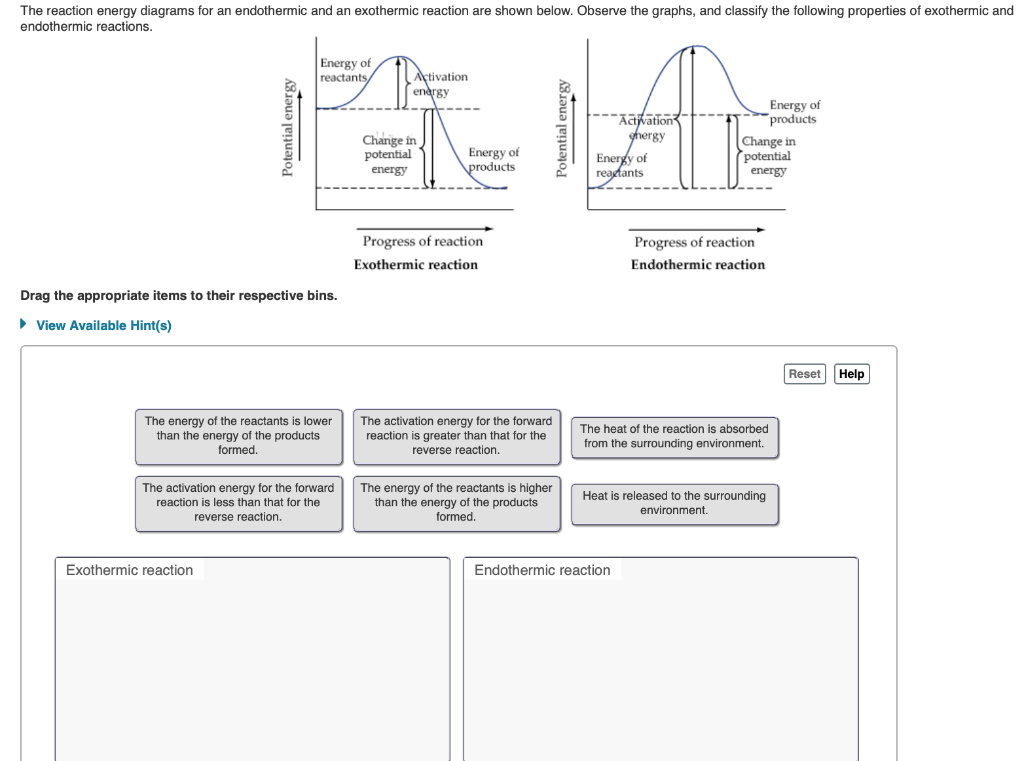
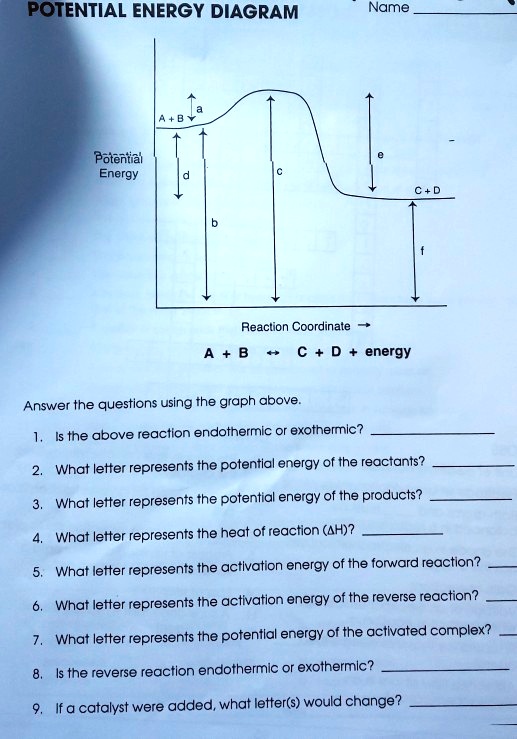
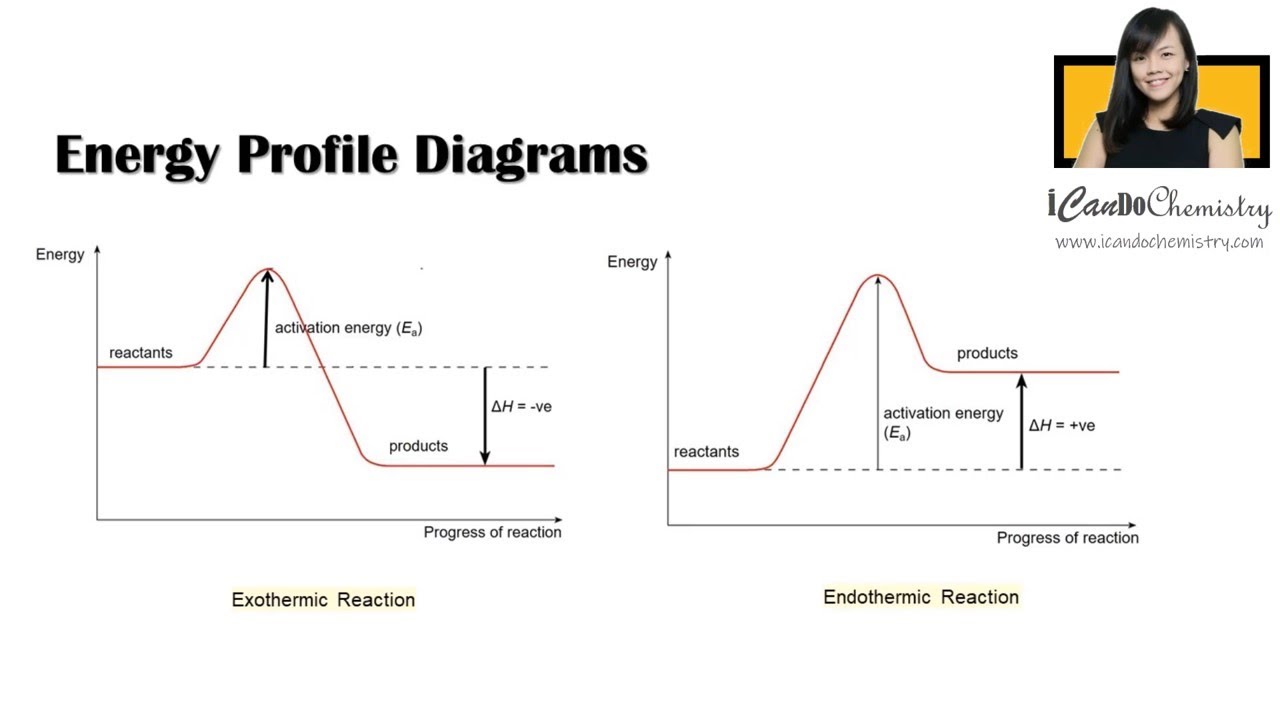
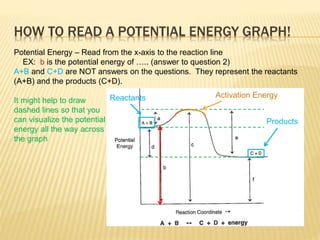
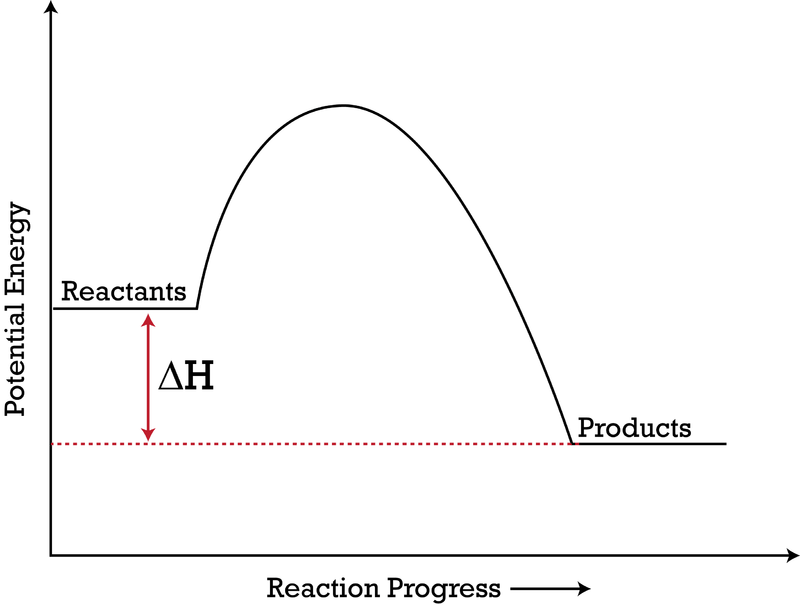
POTENTIAL ENERGY DIAGRAMS the diagrams graphically compare reactant energy to product energy as reaction occurs if reactants have more energy then products, the reaction is exothermic (loss of energy) if reactants have less energy then products, the reaction is endothermic (gain of energy) 18.4: Potential Energy Diagrams - Chemistry LibreTexts Feb 13, 2022 · A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for ...
Columbia | SIPA Center on Global Energy Policy | Low ... 8.3.2021 · Read the Article. Download the PowerPoint. Abstract. Given increased urgency to transition the global economy to net-zero CO2 emission, governments and industry have increased focus on decarbonizing hard-to-abate sectors, including steel making, which contributes roughly 6% of global CO2 emission and 8% of energy related emission (including power …
Potential energy diagram endothermic
Potential Energy Diagrams | CK-12 Foundation 9 Jul 2019 — Figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) ... 18.3 Potential Energy Diagrams - CK-12 23 Feb 2012 — Lesson Objectives. The student will: define internal energy. describe and draw the difference between endothermic and exothermic potential ... The Energy in Chemical Reactions: Thermodynamics and ... Objects can also possess potential energy. An object has potential energy because of its position in relation to other objects. When an object rises against the force of gravity, it gains potential energy; when it falls, it loses potential energy. The object stores this energy as it rises and releases it as it returns to Earth.
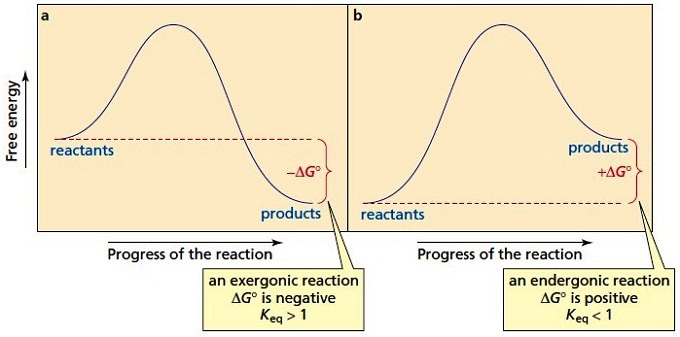
Potential energy diagram endothermic. Potential Energy Diagrams - Chemistry - Catalyst, Endothermic ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Gibbs Free Energy - Purdue University Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.. When a reaction is favored by both enthalpy (H o < 0) and entropy (S o > … Potential Energy Diagrams Chemistry Catalyst Endothermic ... Potential energy diagrams chemistry catalyst, endothermic & exothermic reactions. this chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. it also shows the this chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding in ... Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
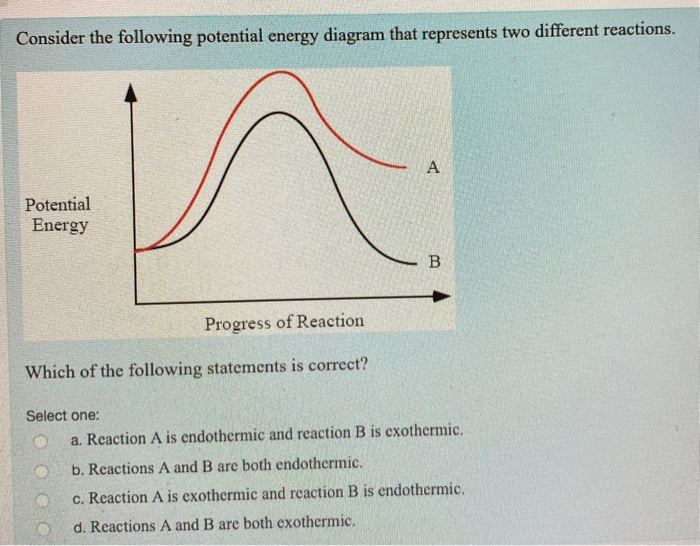
Potential Energy Diagrams | Chemistry for Non-Majors Describe the potential energy diagram for an endothermic reaction. Describe the potential energy diagram for an exothermic reaction. Examples of Chemical Energy in Everyday LIfe 9 Examples of Chemical Energy. Chemical energy is either released (exothermic reaction) or absorbed (endothermic reaction) during a chemical reaction. In an exothermic reaction, heat is released, creating warmth. In an endothermic reaction, the heat is absorbed, creating cooling. Air bags are activated by a chemical reaction inside the bag. How to tell if a reaction is exothermic or endothermic ... Image of a graph showing potential energy in relation to the process of a chemical reaction. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. In other words, the products are more stable than the reactants. What are Endothermic Reactions? (with Examples & Video) Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ...
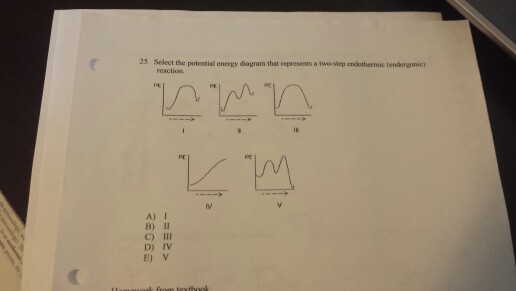
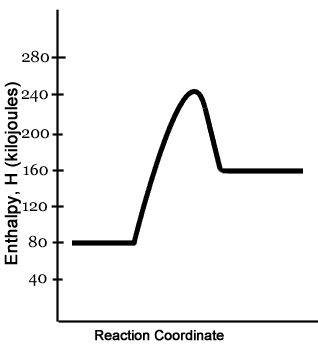
Chemistry 30 Assessment Exemplars, 2020-2021 - Alberta A. endothermic, and the products have more potential energy than the reactants B. endothermic, and the reactants have more potential energy than the products C. exothermic, and the products have more potential energy than the reactants D. exothermic, and the reactants have more potential energy than the products Answer: D Outcome: A1.10k, A2.2k ... Ionization energy - Wikipedia Ionization energy of atoms, denoted E i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound atomic electrons.The measurement is performed in the gas phase on single atoms. While only noble gases occur as monatomic gases, other gases can be split into single atoms. Endothermic Reactions: Definition, Example, Diagram and ... Sep 02, 2021 · Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. Energy - Wikipedia Energy is also transferred from potential energy to kinetic energy and then back to potential energy constantly. This is referred to as conservation of energy. In this isolated system , energy cannot be created or destroyed; therefore, the initial energy and the final energy will be …
The Energy in Chemical Reactions: Thermodynamics and ... Objects can also possess potential energy. An object has potential energy because of its position in relation to other objects. When an object rises against the force of gravity, it gains potential energy; when it falls, it loses potential energy. The object stores this energy as it rises and releases it as it returns to Earth.
18.3 Potential Energy Diagrams - CK-12 23 Feb 2012 — Lesson Objectives. The student will: define internal energy. describe and draw the difference between endothermic and exothermic potential ...
Potential Energy Diagrams | CK-12 Foundation 9 Jul 2019 — Figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) ...



















0 Response to "38 Potential Energy Diagram Endothermic"
Post a Comment