40 Sf6 Molecular Orbital Diagram
Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. › 40267778 › General_chemistry_5th(PDF) General chemistry 5th edition | MOHD DHAIBAN - Academia.edu (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
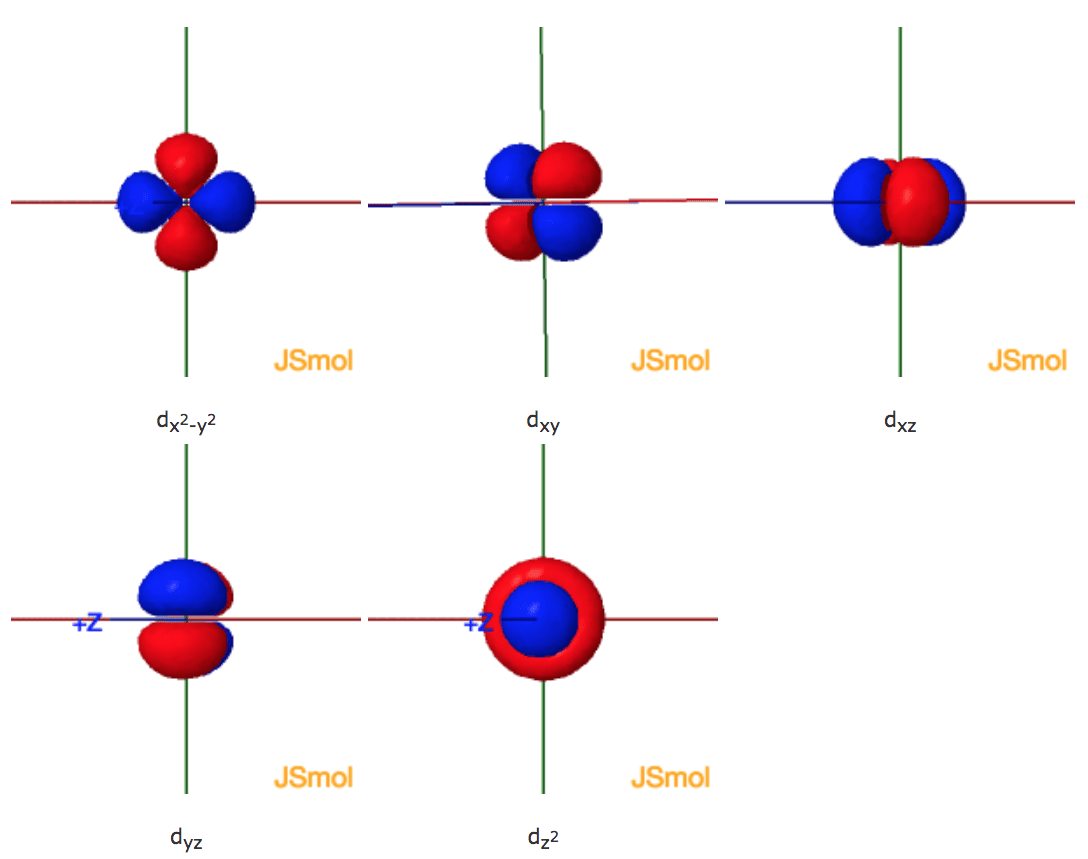
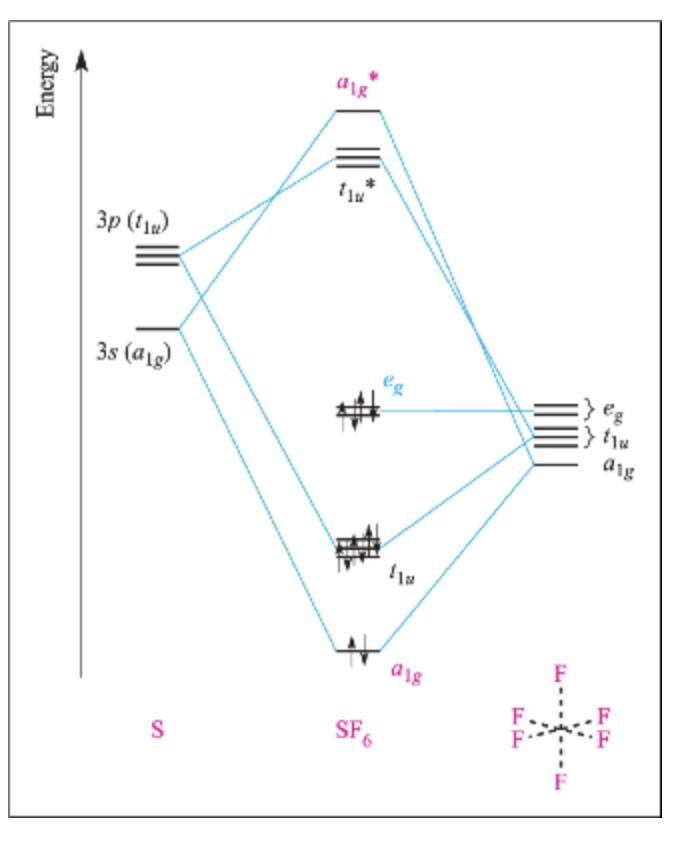
Answer in Inorganic Chemistry for Olebogeng #141937 2 Nov 2020 — In the construction of the molecular orbital diagram of a polyatomic molecule (SF6), 12 valence electrons are used to occupy 10 MO; ...1 answer · Top answer: Solution. In the SF6 molecule, 6 electrons from sulfur and 1 electron from six fluorine atoms are involved. A total of 12. 4 binding, 2 non-binding ...
Sf6 molecular orbital diagram
Bonding in Molecules Molecular orbital diagrams for hyper-coordinate molecules. 15. The bonding in XeF2 (and CO2). 16. 12-electron main group octahedral systems: SF6 as an ...71 pages 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. However, with more atoms, computers are required to calculate how the atomic orbitals combine. PDF Molecular Geometry and Bonding Theories Chapter 9 CHEMA1301 Molecules with five or six electron domains around the central atom have molecular geometries based on Predict whether these molecules are polar or nonpolar: (a) BrCl, (b) SO2, (c) SF6. a. Br-Cl is a Molecular orbital theory correctly predicts that hydrogen forms diatomic molecules but helium does...
Sf6 molecular orbital diagram. ebin.pub › 43-years-jee-advanced-1978-2020-jee43 Years JEE ADVANCED (1978-2020) + JEE MAIN Chapterwise & ... Which of the following best describes the diagram of molecular orbital? [Main Online April 15, 2018 (II)] (a) (b) (c) (d) 14. A bonding π orbital A non-bonding orbital An antibonding σ orbital An antibonding π orbital Which of the following is paramagnetic ? [Main Online April 8, 2017] (a) NO+ (b) CO (c) (d) B2 15. Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals. Have you ever thought about how sigma and pi bonds are formed? What is the difference between diamagnetic and paramagnetic behaviour? SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity SF6 is an inorganic gas which is used widely in the production of sulphuric acid and sulphurs. This is because it is an exception to the octet role and can expand its orbital to accommodate more electrons. SF6 Molecular Geometry. When we look at Sulphur Hexafluoride molecule, Sulphur is... PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Accordingly, a molecular orbital diagram such as Figure 9-5 is inappropriate for heteronuclear diatomic molecules. If the two elements are similar (as in NO or CN mole-cules, for example), we can modify the diagram of Figure 9-5 by skewing it slightly.
Paramagnetic vs Diamagnetic | Forum To answer the question you have to draw the molecular orbital diagram or electron configuration for OF+ and NO+ (not the lewis For a molecule to be paramagnetic, there has to be at least one unpaired electron in a molecular orbital. Molecular Orbital Diagrams -- Chemistry X - YouTube For chem videos, quizzes and more download Chemistry X for free on the App Store! Correlation Diagrams - by considering the positions and energies of... Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or... Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
Molecular Orbitals in SF6 Molecular Orbitals in SF6 The Lewis structure of SF6 describes six pairs of electrons as bond pairs, despite the availability of only four valence orbitals on sulphur. The valence bond description of this hypervalent complex must therefore invoke d-orbitals. However, high-level theoretical calculations... Molecular Orbital Theory - Chemistry | Socratic Molecular orbital theory is a method for determining molecular structure. It describes electrons as moving under the influence of the nucleus and not Molecular Orbital (MO) theory better explains the properties of more complex molecules. MO theory explains the partial bonds of NO₃⁻ without using... › 29794507 › Solid_State_ChemiStrySolid State ChemiStry and itS appliCation 2014 ... - Academia.edu Solid State ChemiStry and itS appliCation 2014 Anthony R. West › tfile › studymaterialNCERT Xtract - Objective Chemistry.pdf - Chemistry Class 11 ... The orbital diagram in which the Aufbau principle is violated, is :, 2s, 2p, (a), (b), (c), (d), 120. If n = 6, the correct sequence for filling of electrons will be :, (a) ns (n – 2) f (n – 1) d np, (b) ns (n – 1) d (n – 2) f np, (c) ns (n – 2) f np (n – 1) d, (d) ns np (n – 1) d (n – 2) f, 121.
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... Lecture 4 Revision of hybridisation Molecular orbital theory and diatomic molecules. BeH2 BF3, CO32-C2H4 SO42-, CH4, NH3, H2O, PCl5, SF4. SF6 [Ni(CN)4]2-[PtCl4]2-. 66. • Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation (these are...
Molecular Orbital Theory (5.4) - Chemistry 110 | Chapter Six Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons. Draw the molecular orbital diagram for the oxygen molecule, O2. Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here.
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
pubs.acs.org › doi › 10Polymer Adhesion: Seeking New Solutions for an Old Problem ... Nov 28, 2021 · Polymer adhesion is ubiquitous in both the natural world and human technology. It is also a complex multiscale phenomenon, such that the solution of adhesion problems requires a convergence of chemistry, physics, and engineering. In this Perspective, we provide an overview of some of the fundamental concepts that have emerged in the field of polymer adhesion, discuss recent work, and identify ...
Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the One of the molecular orbitals in this molecule is constructed by adding the mathematical functions The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
PDF Microsoft Word - Chapter 1_6_SY.doc Molecular Orbital Diagrams (H2 and He2) One of the strengths of molecular orbital theory is its ability to describe the energy of both occupied and unoccupied molecular orbitals for a molecule. A molecular orbital diagram shows both the energy of the atomic orbitals (from the atoms that are...
Molecular Orbital Theory: Types, Methods, Rules, Examples and... Molecular Orbital Theory. We know that atoms bond. That results in the diversity of matter around us. According to the Molecular Orbital Theory, individual atoms combine to form molecular orbitals. Thus the electrons of an atom are present in various atomic orbitals and are associated with several...
8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O2. Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here.
SF6 Lewis Structure, Molecular Geometry, Hybridization, and ... SF6 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are ...4 Mar 2021 · Uploaded by Eric Victor
PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... Polyatomic Molecular Orbital Theory. Transformational properties of atomic orbitals. • When bonds are formed, atomic orbitals combine according to their symmetry. MO diagram of homonuclear diatomic molecules.
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Molecular Orbital Diagrams simplified. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory.
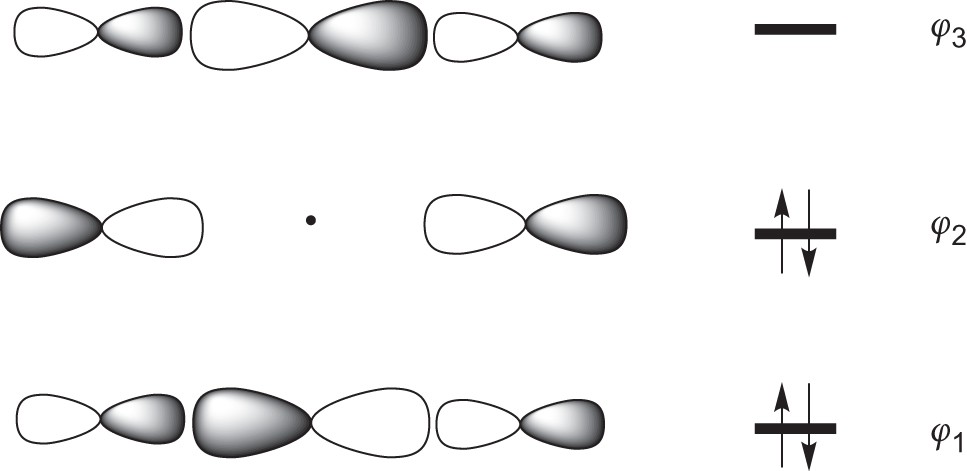
Energy level diagram for Molecular orbitals - Chemical Bonding and... The first ten molecular orbitals may be arranged in order of energy as follow 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals.
PDF Lecture 1 Recap of molecular orbital theory. 18-electron rule. Lecture 3: Ligand classes. s-donor complexes. Octahedral ML6 molecular orbital energy diagram. Lecture 5: p-donor ligands, metal-ligand multiple bonds, O2-, R2N-, RN2-, N3-. Lecture 6: ML6 molecular orbital energy diagrams incorporating...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
PDF PowerPoint Presentation u Stable electronic configurations: MO Energy Level Diagrams Reviewed u Electron count preference u Electron count and Oxidation States u Ligands. • Carbon Monoxide • Phosphines • Cyclopentadienide and arenes • Hydrides and dihydrogen.
Molecular Orbital Diagram: study guides and answers on Quizlet Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. ( i have no faqing idea man, how do u Place the following substances in order of increasing vapor pressure at a given temperature. a. SiH4 < SF4 < SF6 b. SF4 < SF6 < SiH4 c. SF6 < SiH4 < SF4 d...
Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2+.
What is the molecular orbital diagram for O2- and O2+ ions? - Quora Does molecular orbital theory talk about the colour of resultant molecule? The first photo is straight from a 2006 edition Pearson general chemistry textbook, and it shows you what the molecular orbital (MO) diagram for O2 is.
MO lecture #3 Molecular Orbital Diagrams of more complicated molecules. √. √ z y x. First let's consider the sulfur orbitals ... in the more specific case of SF6, these.10 pages
CO2 MOs Molecular Orbitals in SF6. The Lewis structure of SF6 describes six ... MO Diagram for SF6. S 3p. S 3s. SF6 MOs bonding MOs antibonding MOs non-bonding.3 pages
pubs.acs.org › doi › 10Selective Detection of Carbon Monoxide on P-Block Doped ... Jan 19, 2022 · CO and CO2 are among the most commonly monitored gases. However, the currently available semiconductor sensors require heating to ∼400 °C in order to operate effectively. This increases the power demand and shortens their lifespan. Consequently, new material prospects are being investigated. The adoption of novel two-dimensional layered materials is one of the pursued solutions. MoS2 and ...
Chapter 6 - Molecular Structure Chapter 6 - Molecular Structure. Introduction. A method for constructing Lewis structures of simple molecules and ions The group number of sulfur is 6 and its oxidation state in SF4 is +4, so the In molecular orbital theory, atomic orbitals on different atoms are mixed to produce bonds that can be...
PDF Molecular Geometry and Bonding Theories Chapter 9 CHEMA1301 Molecules with five or six electron domains around the central atom have molecular geometries based on Predict whether these molecules are polar or nonpolar: (a) BrCl, (b) SO2, (c) SF6. a. Br-Cl is a Molecular orbital theory correctly predicts that hydrogen forms diatomic molecules but helium does...
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. However, with more atoms, computers are required to calculate how the atomic orbitals combine.
Bonding in Molecules Molecular orbital diagrams for hyper-coordinate molecules. 15. The bonding in XeF2 (and CO2). 16. 12-electron main group octahedral systems: SF6 as an ...71 pages
![How is the bonding in the [Au6C(PPh3)6]2+ cluster explained ...](https://i.stack.imgur.com/Y1psc.png)

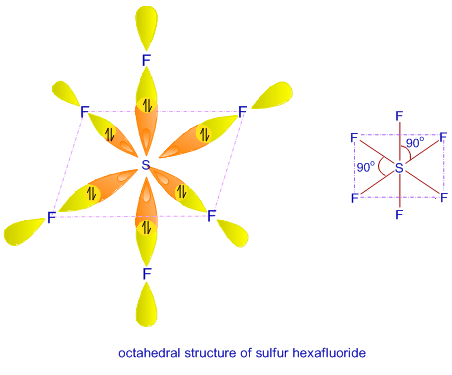






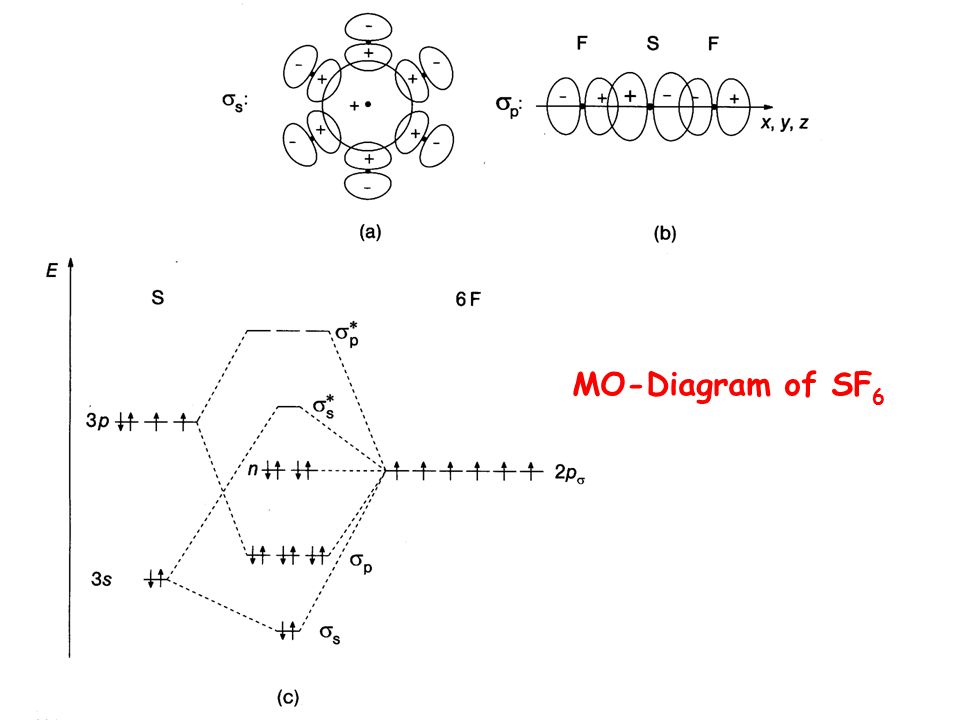




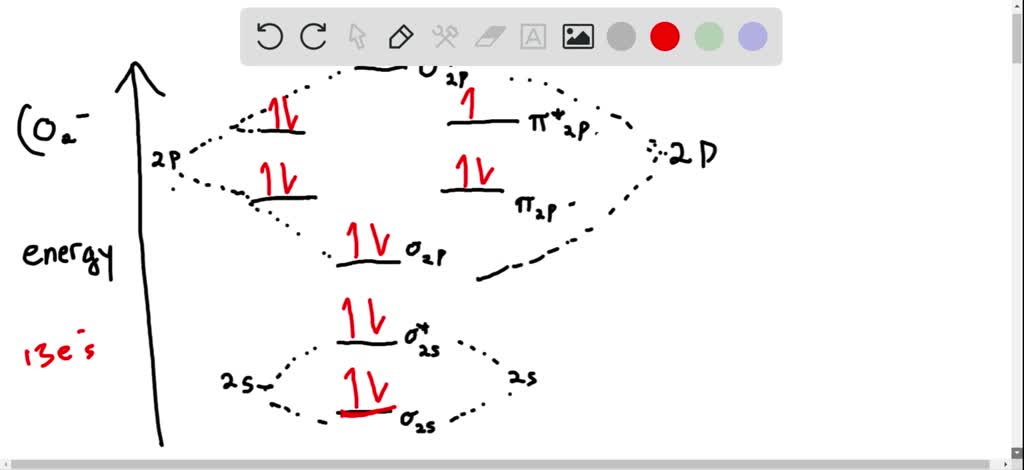
0 Response to "40 Sf6 Molecular Orbital Diagram"
Post a Comment