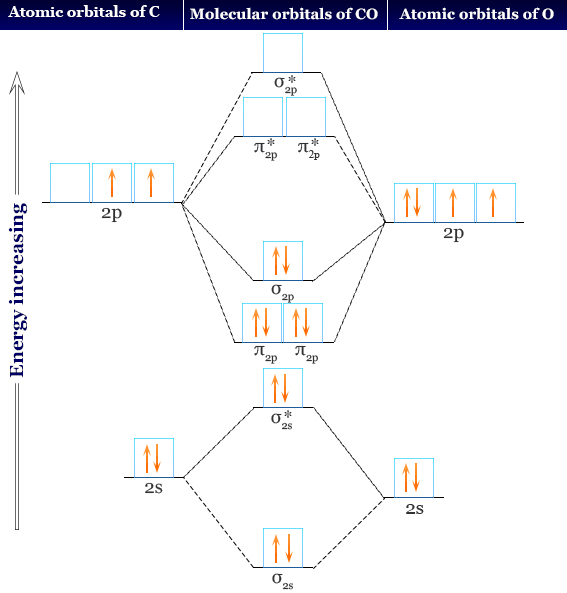
41 Molecular Orbital Diagram For Co
Cyanide Molecular Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. In this answer of Martin's, you can find a molecular orbital diagram of $\ce{CO}$. By writing molecular orbital configuration for NO,CO,O2 ... You can see that CO is not (as it has zero unpaired electrons), but NO is (it has one unpaired electron). Well, the MO diagram for O2 is: The bond order is already calculated in the diagram. The bonding electrons are in the σ2s, σ2p, π2px, and π2py MOs, giving 2 + 2 + 2 + 2 = 8.
Carbon Orbital diagram, Electron configuration, and ... The orbital diagram for Carbon is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Carbon orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest two electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Carbon atom is shown below-.
Molecular orbital diagram for co
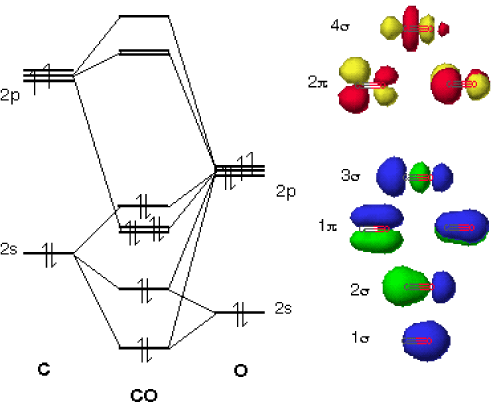
What is the molecular orbital energy diagram of CO? - Quora Answer (1 of 4): Electronegativity - Chemistry LibreTexts The electrons are actually in a molecular orbital, and are moving around all the time within that orbital. This sort of bond could be thought of as being a "pure" covalent bond - where the electrons are shared evenly between the two atoms. What if B is slightly more electronegative than A? B will attract the electron pair rather more than A does. That means that the B end of … How To Draw Molecular Orbital Diagram Of Co - Drawing ... Co molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).according to molecular orbital diagram, molecular orbital configuration is given as σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰ Label orbitals sigma, sigma*, pi or pi*.
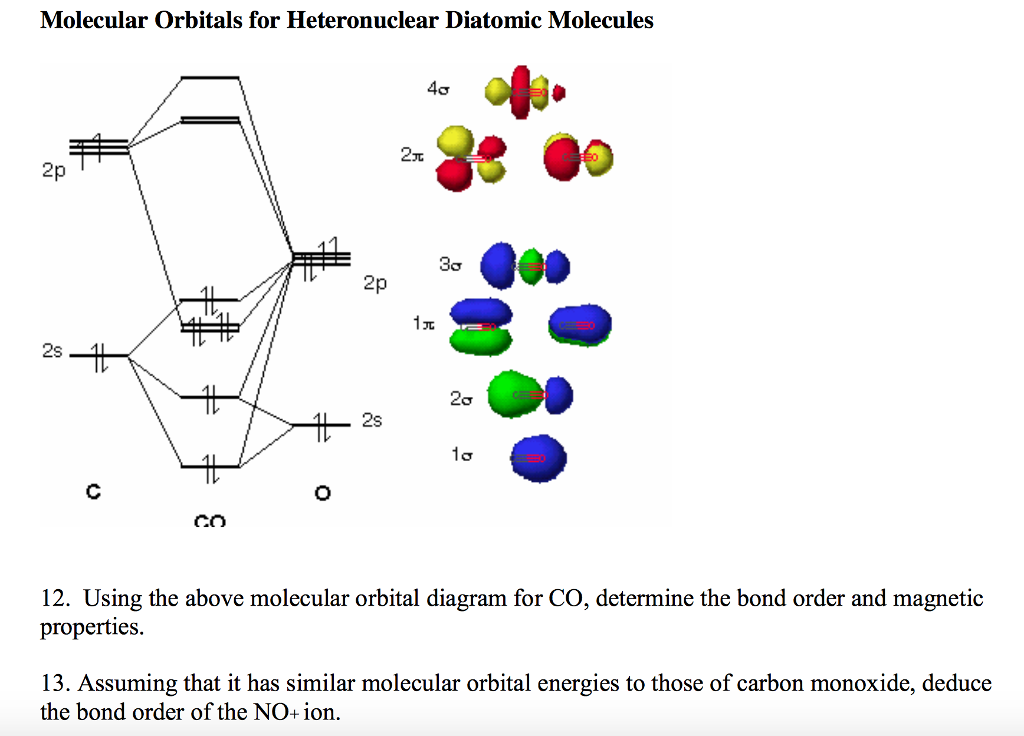
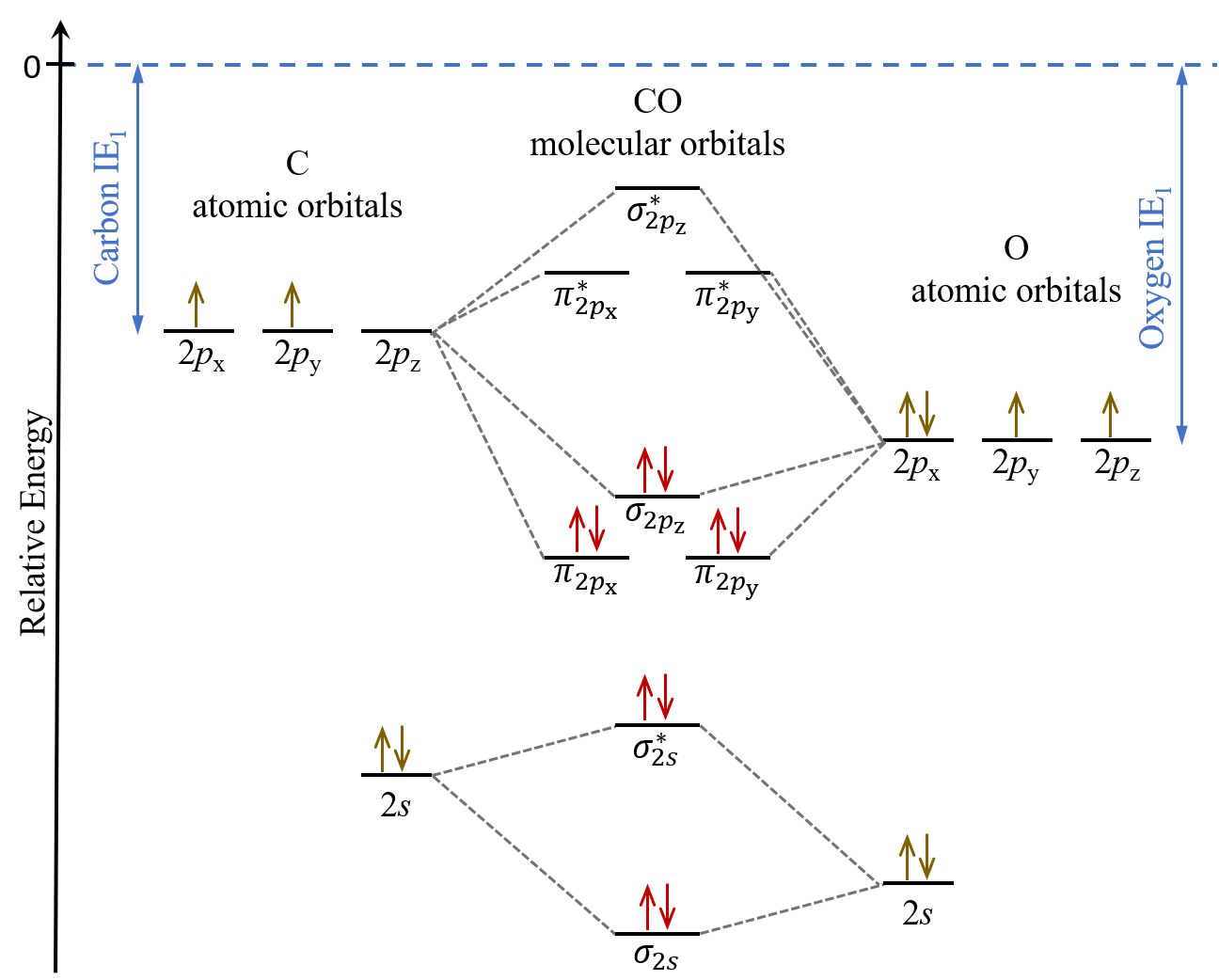
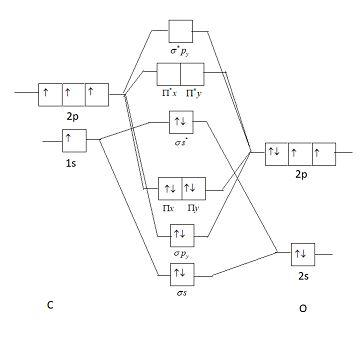
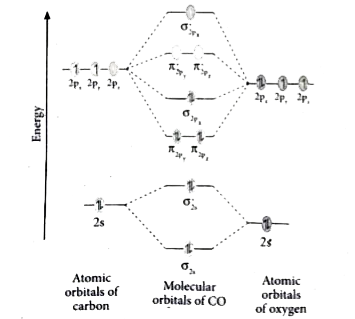
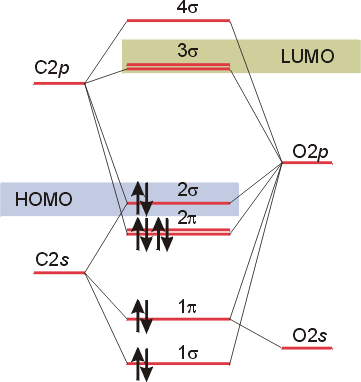
Molecular orbital diagram for co. PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules together to produce a sigma molecular orbital [σ = (1sa + 1sb)]. Since the electrons in this orbital are more stable than on the individual atoms, this is referred to as a bonding molecular orbital. A second molecular orbital is also created, which we simplistically show as a subtraction of the two atomic 1s orbitals [σ* = (1sa - 1sb)]. This ... Bonding in Metal Carbonyls: Explaination, Type, Property ... Molecular Orbital Diagram of CO. To understand the bonding in metal carbonyls, we need to first learn the Molecular Orbital \(\left( {{\rm{MO}}} \right)\) diagram of carbon monoxide. There are ten electrons in the carbon monoxide ligand. The order of energy of the molecular orbitals and the accommodation of ten electrons of the carbon monoxide ... Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine … orbitals - How to rationalise with MO theory that CO is a ... The electrons in the frontier orbital (s) play a special role for the chemical reactivity. In C O, the HOMO is the 5 σ orbital (ref: your diagram), and has mainly C character. The two electrons in it act like a lone pair on the carbon. The predominant C character of the HOMO accounts for the reactivity via carbon ( σ donor
Molecular Orbital diagram for CO - Ultraviolet and Visible ... Energy levels: Molecular Orbital Theory - revision 11:10. Molecular Orbital diagram for CO 5:09. Taught By. Patrick J O'Malley, D.Sc. Reader. Try the Course for Free. Transcript. Explore our Catalog Join for free and get personalized recommendations, updates and offers. ... What is the molecular orbital for CO? – Leagueslider.com Molecular orbital diagram of carbon monoxide On the molecular orbital model of carbon monoxide, the two spx hybrid orbitals of oxygen and carbon combine to give two molecular orbitals. One is low energy sigma bonding type contains two electrons and the other is high energy sigma antibonding type. What is the molecular orbital order of CO? Master Organic Chemistry - An Online Organic Chemistry ... 09.11.2021 · 400+ free articles on undergraduate organic chemistry topics plus free (and paid) study guides, a reaction encyclopedia, practice problems, tutoring & more. What is the molecular orbital energy diagram of CO? - Quora Answer (1 of 4):
Molecular Orbital Theory - Detailed Explanation with ... The Molecular Orbital Theory (often abbreviated to MOT) is a theory on chemical bonding developed at the beginning of the twentieth century by F. Hund and R. S. Mulliken to describe the structure and properties of different molecules. The valence-bond theory failed to adequately explain how certain molecules contain two or more equivalent bonds ... What is molecular orbital diagram of CO? – handlebar-online.com Nov 28, 2020 · What is molecular orbital diagram of CO? Carbon monoxide MO diagram. Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row elements. The valence molecular orbitals in both atoms are the 2s and 2p orbitals. The molecular orbital diagram for carbon monoxide (Figure 5.3. Is there SP mixing in CO? Molecular Orbitals - an overview | ScienceDirect Topics The molecular orbital wave function, ignoring normalization, is (21) Φ g g MO ( 1 , 2 ) = ϕ g ( 1 ) ϕ g ( 2 ) = [ χ 1 χ r + χ r χ 1 ] + [ χ 1 χ 1 + χ r χ r ] , whereas the valence bond wave function is Molecular Orbitals for Carbon Monoxide Molecular Orbitals for CO. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital in the energy level correlation diagram shown The results displayed may be switched between those from a low level of calculation and those from a high level.
Molecular orbital energy diagram of CO+ - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Carbon Monoxide Molecular Orbital Diagram Explanation A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. . combinations such as CO and NO show that the 3σg MO is higher in energy. Mulliken came up with theory known as Molecular Orbital Theory to explain questions like above.
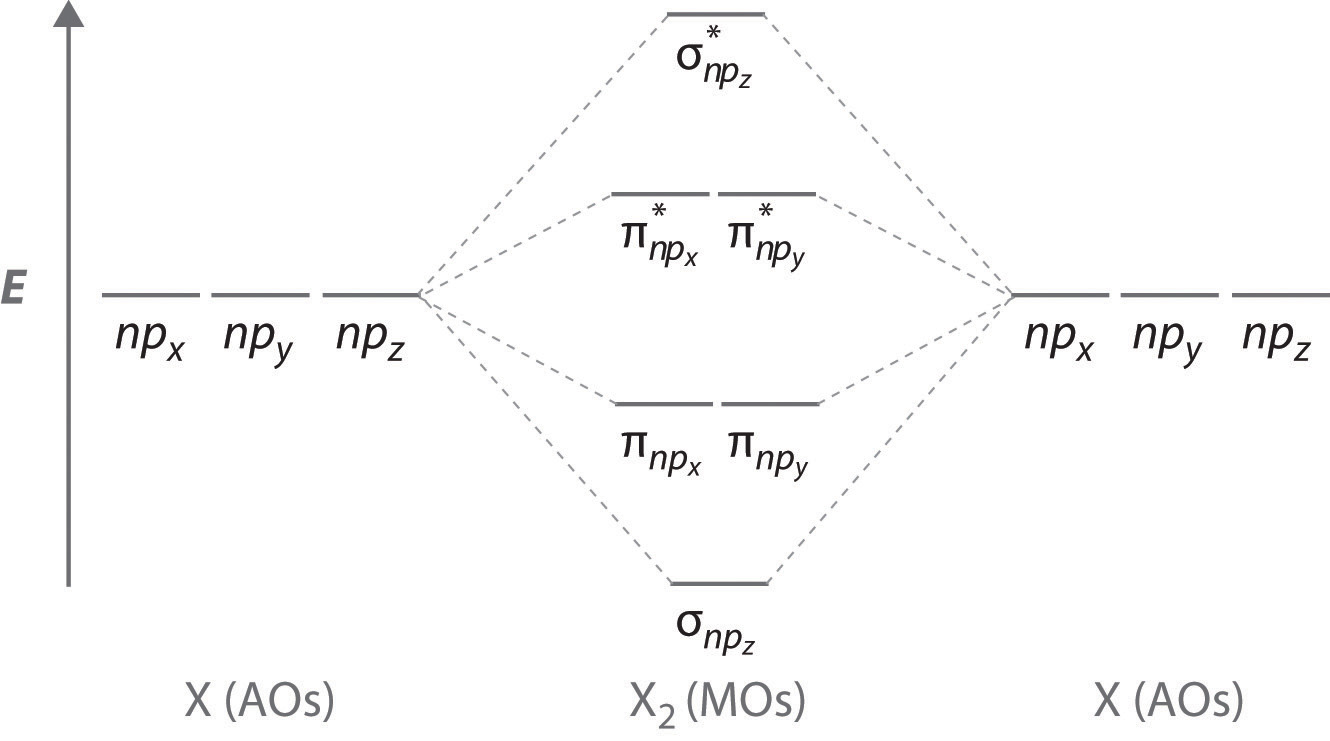
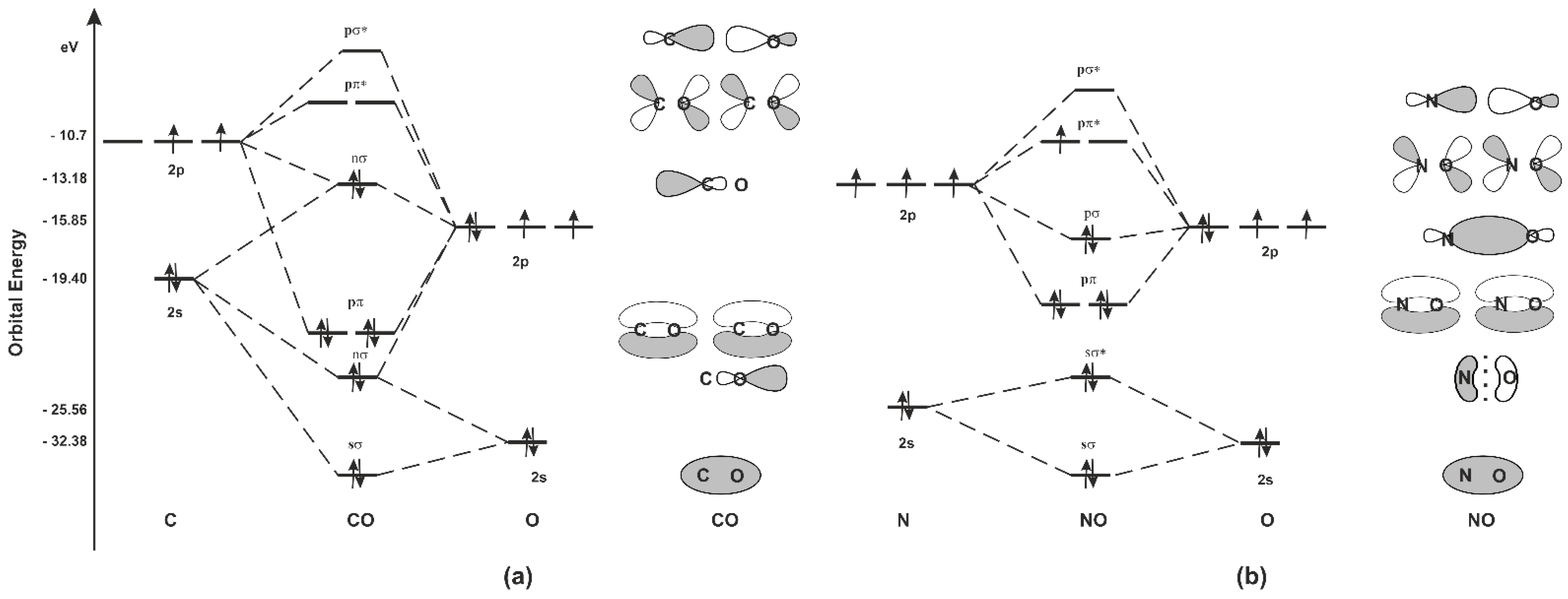
N2+ Mo Diagram - schematron.org 03.02.2019 · Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Molecular Orbital Theory – Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the …
Molecular Orbital Theory (MOT), Chemistry Study Material ... For Example, if we look at CO Molecule, it is diamagnetic as all the electron in CO are paired as in the figure below: Fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
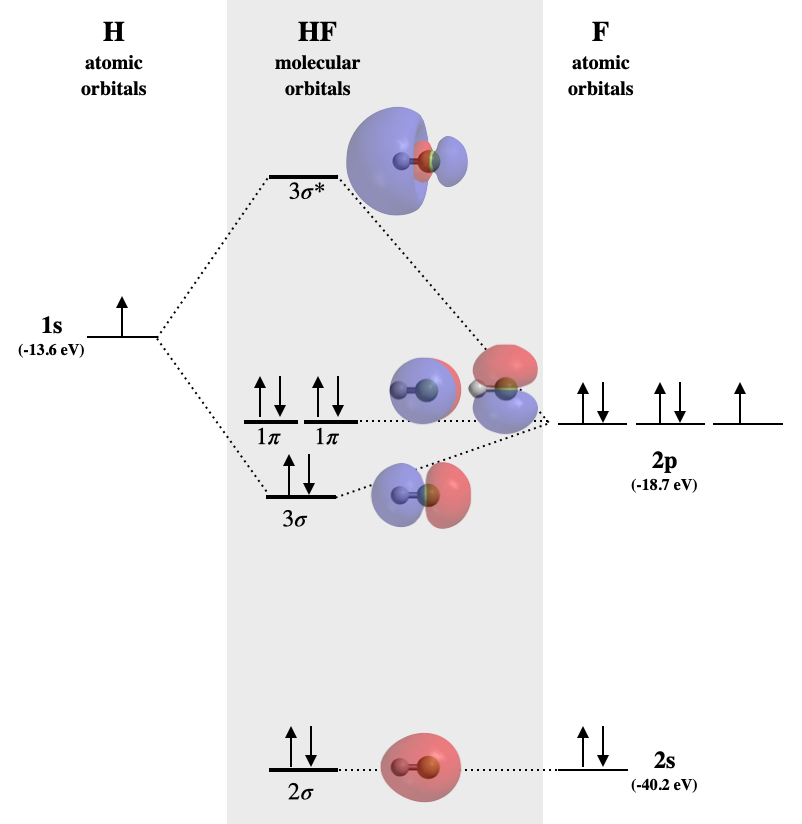
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
PDF Simple Molecular Orbital Theory - University of California ... LCAO MO Energy Diagram for H2 Energy H-H ∆E1 ∆E2 • ∆E2> ∆E1, so the antibonding orbital is always more anti-bonding than the bonding orbital is bonding H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. Ha Hb
Molecular Orbital Diagram of CO - All About Chemistry 1. 246. Molecular Orbital Diagram of CO. TAGS. Molecular Orbital Diagram. Previous article Wohl-Ziegler Bromination. Next article Molecular Orbital Diagram of NO.
inorganic chemistry - Molecular orbital diagram of CO and ... Molecular orbital diagram of CO and charge localisation. Ask Question Asked 1 year, 8 months ago. Active 1 year, 8 months ago. Viewed 129 times 4 1 $\begingroup$ MO diagram of CO. My question concerns the interpretation of the Molecular Orbital of CO. I think I find it clear how you build it but I have some concerns about how you rationalize it.
Molecular orbitals in Carbon Monoxide - ChemTube3D Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. Hydrogen | Fluorine | Nitrogen | Hydrogen Fluoride | Carbon Monoxide | Methane | Ammonia | Ethylene | Acetylene | Allene | Formaldehyde | Benzene
DOC Molecular Orbital Theory of Octahedral Complexes In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. No Metal- Ligand -bonding ( bonding only) Let's take [Co(NH3)6]3+ as an example. Using the LGO method, one can construct a qualitative MO diagram for bonding in a [ML6]n+ complex.
H.O.M.O (Highest Occupied Molecular Orbital) of CO ... H.O.M.O (Highest Occupied Molecular Orbital) of CO molecular is: A Non-bonding M.O. with slight antibonding character B Non-bonding M.O. with slight bonding character C Pure non-bonding M.O D None of the above Medium Open in App Solution Verified by Toppr Correct option is B) Two atoms come together and interact to form a bond.
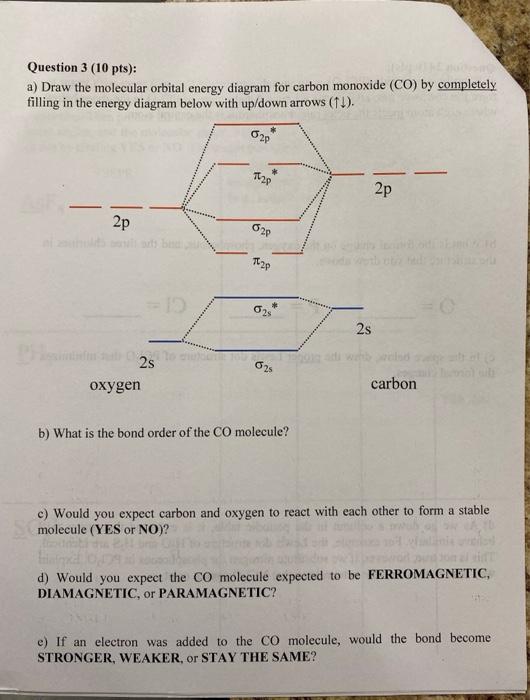
Solved 3) Sketch the molecular orbital diagram for CO ... 3) Sketch the molecular orbital diagram for CO. Calculate the bond order. Identify the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Does the structure predicted by molecular orbital theory match the Lewis Dot Structure? Question: 3) Sketch the molecular orbital diagram for CO. Calculate the bond ...
9.8: Molecular Orbital Theory - Chemistry LibreTexts Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ...
Molecular orbital diagram of CO. | Download Scientific Diagram Contexts in source publication. ... simple molecular orbital (MO) diagram for CO is shown below ( Figure 1 ). The highest occupied molecular orbital (HOMO) is indicated by the pair of electrons ...
9.7: Molecular Orbitals - Chemistry LibreTexts Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
How To Draw Molecular Orbital Diagram Of Co - Drawing ... Co molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).according to molecular orbital diagram, molecular orbital configuration is given as σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰ Label orbitals sigma, sigma*, pi or pi*.
Electronegativity - Chemistry LibreTexts The electrons are actually in a molecular orbital, and are moving around all the time within that orbital. This sort of bond could be thought of as being a "pure" covalent bond - where the electrons are shared evenly between the two atoms. What if B is slightly more electronegative than A? B will attract the electron pair rather more than A does. That means that the B end of …
What is the molecular orbital energy diagram of CO? - Quora Answer (1 of 4):
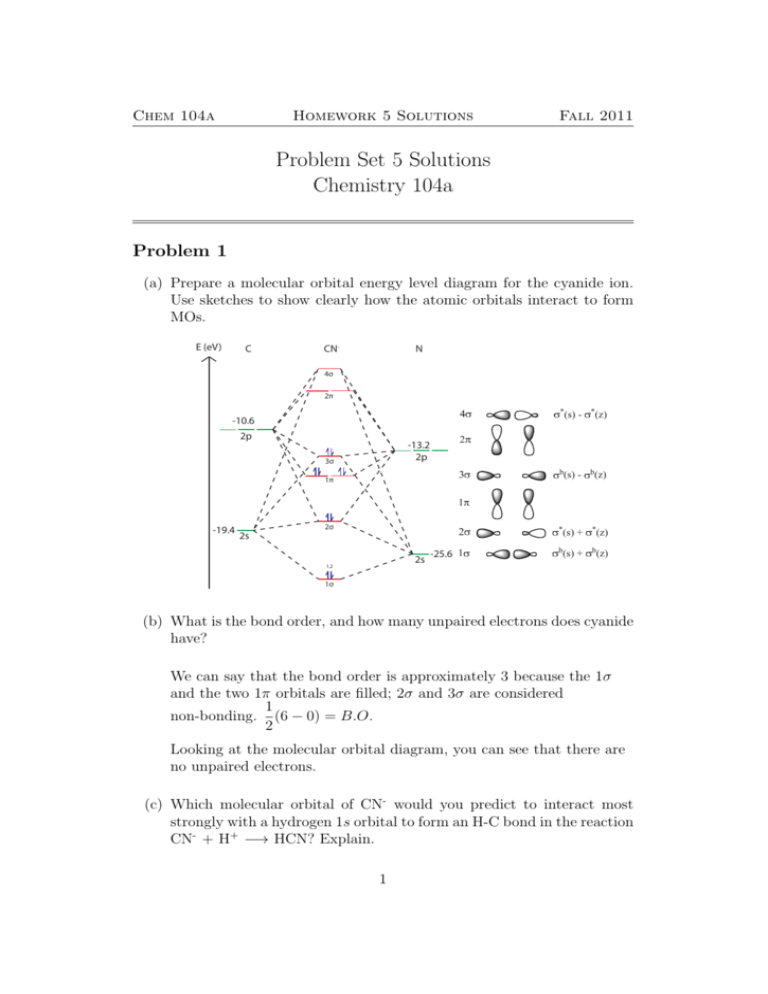















0 Response to "41 Molecular Orbital Diagram For Co"
Post a Comment