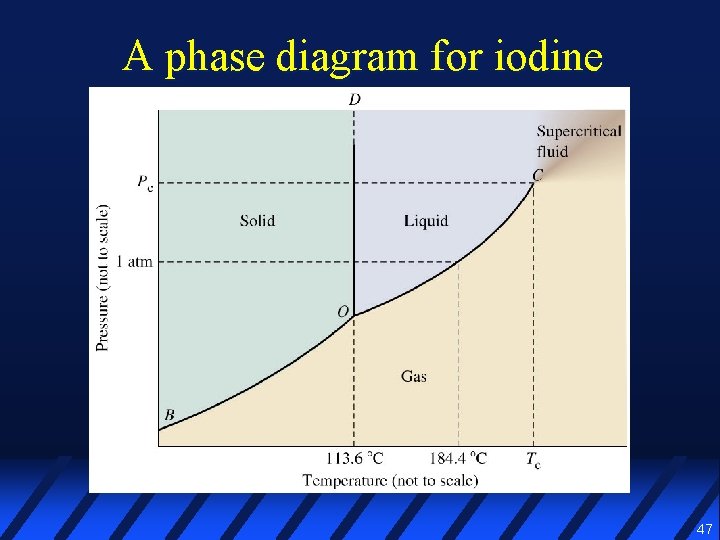
41 phase diagram for iodine
This article from UWaterloo has a phase diagram for iodine, and explains some key points. The vapor pressure of solid Iodine at 20 ∘ C is 0.027 k P a (compare with atmospheric pressure: 101.3 k P a ). If left in an unsealed flask at standard temp/pressure, it will slowly sublime away. But solid iodine does have a melting point, if the flask ... Phase equilibria in the cross sections of isothermal-isobaric sections of the phase diagram of four-component iodine-potassium iodide-water-propyl alcohol are investigated at 298.15 K and pressure of 101325 Pa. It is shown that a three-phase equilibrium of the eutonic type occurs in the cross sections containing (I) 10 and (II) 30 wt % of propyl alcohol, and two three-phase equilibria of the ...
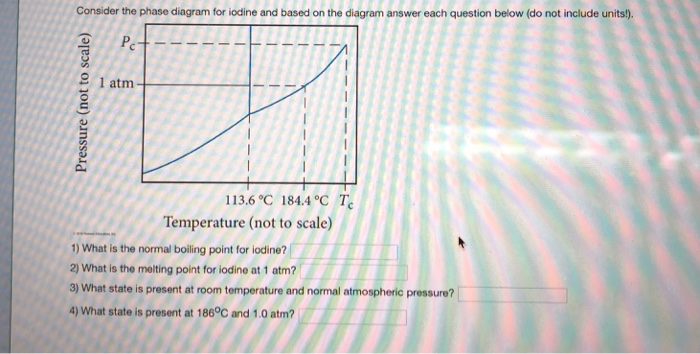
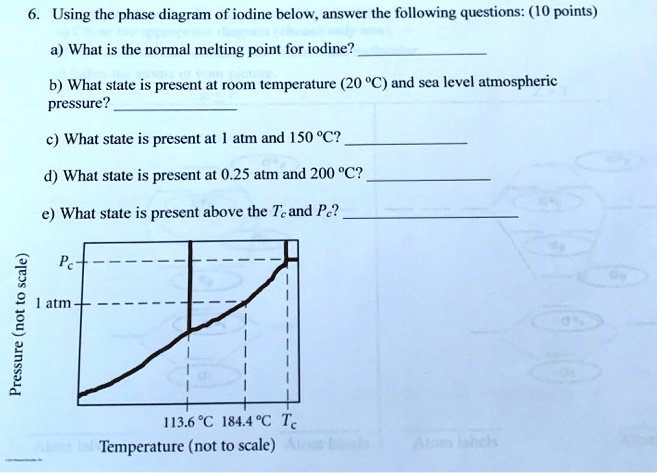
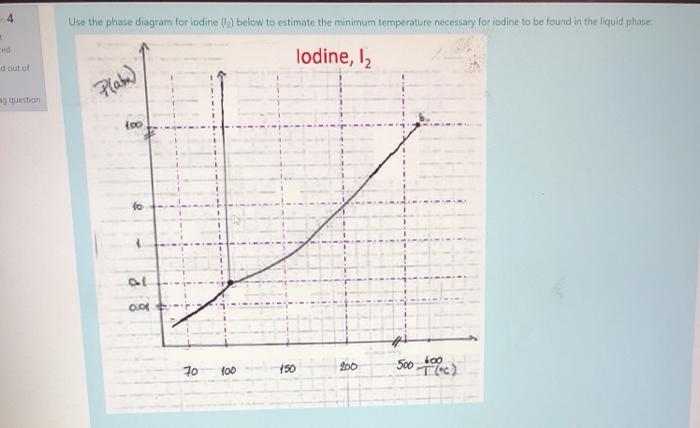
Consider the phase diagram for iodine shown below and answer each of the following questions (Figure 1) What is the melting point for iodine at 1 atm? Express your answer in degrees Celsius using four significant figures. Learn this topic by watching Phase Diagrams Concept Videos. All Chemistry Practice Problems Phase Diagrams Practice Problems.
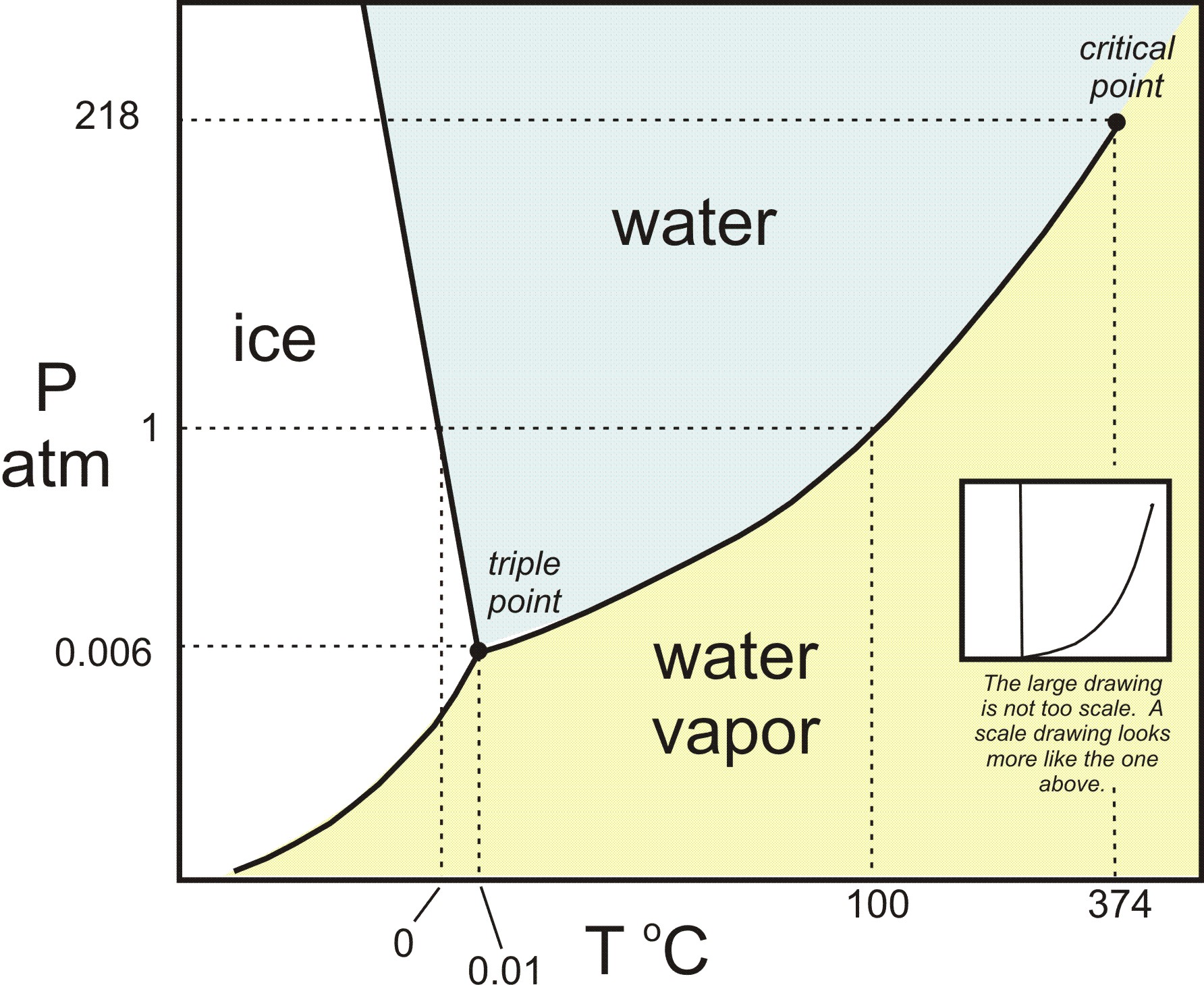
Phase diagram for iodine
Inconsistent explanations, then, can serve as a basis for developing misconceptions and preconceptions in latter students' education. Thus, the notion that upon heating iodine only sublimes, but does not melt is present in many chemistry textbooks, teachers lectures and, therefore, in students ... Consider the phase diagram for iodine shown here and answer each of the following questions. a. What is the normal boiling point for iodine? b. What is the melting point for iodine at 1 atm? c. What state is present at room temperature and normal atmospheric pressure? d. What state is present at $186{ }^{\circ} \mathrm{C}$ and $1.0 \mathrm{~atm}$ ? September 1, 2012 - Phase equilibriums are studied in the isothermal-isobaric sections of the phase diagram of a fourcomponent iodine-potassium iodide-water-ethanol system at 25°C and atmospheric pressure. The compositions of the solvent at which it exhibits the greatest ability to dissolve iodine are established.
Phase diagram for iodine. Phase diagram of iodine. Another useful type of phase diagrams is the binary mixture phase diagram, usually showing temperature versus concentration at constant pressure. These diagrams are more useful than the pressure-concentration diagrams, since it is easier to control temperature than to achieve pressure control. And there's a couple cars. This question. It asked us to consider the phase diagram for I iodine, which is shown at the top of the next page in your textbook. And I have it shown right here so we can see a couple things. Just looking at this graph, which is not trying to scale. PDF | The melt phase encapsulation of gadolinium iodide (GdI3) in small internal diameter carbon nanotubes (CNT) was explored to understand how the... | Find, read and cite all the research you ... Diatomic iodine phase diagram 12 . [...] This paper deals with the results of an ongoing activity carried out by SITAEL and UniPi aimed at the development of technologies for iodine-fed Hall ...
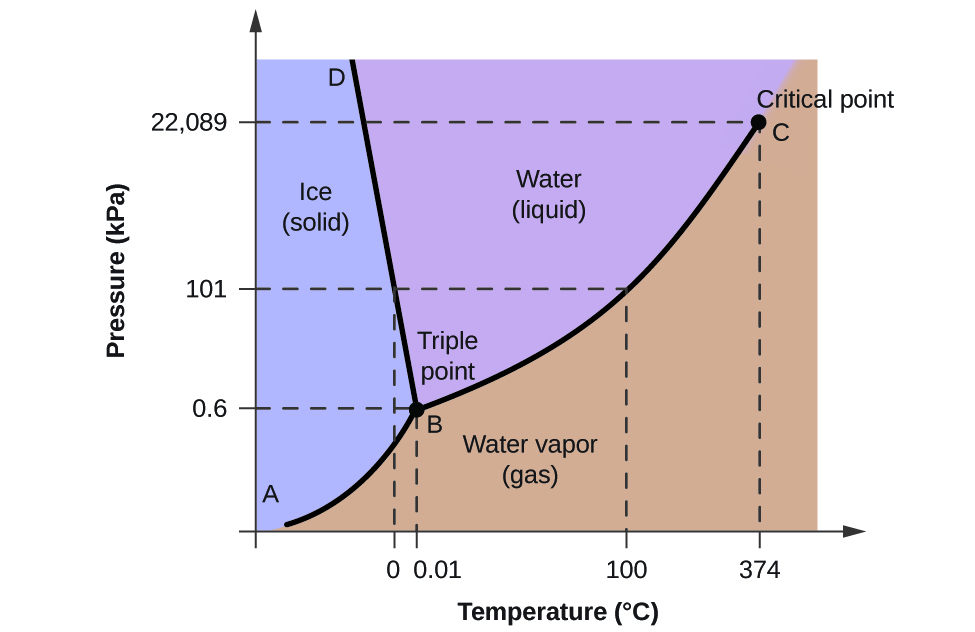
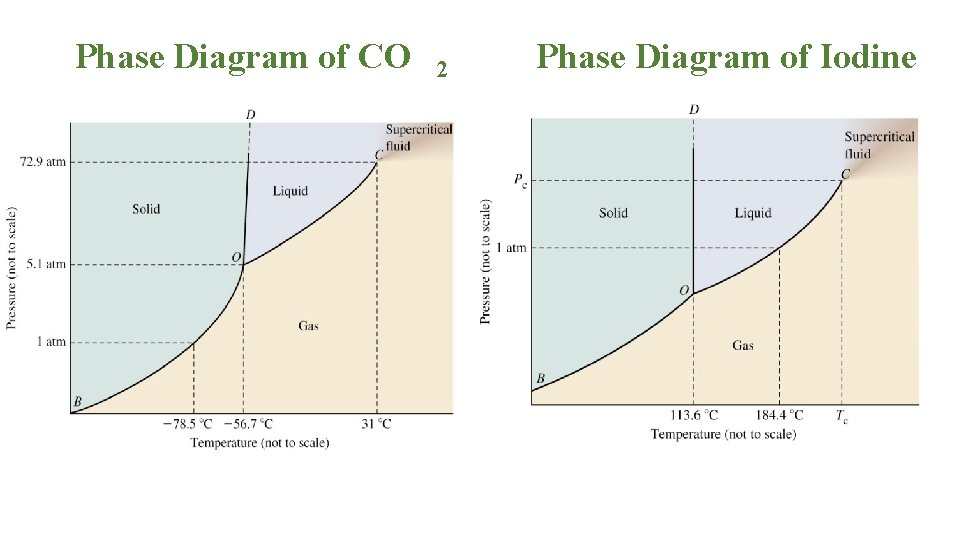
The Bismuth Iodide-Iodine Phase Diagram1 ... Growth of bismuth tri-iodide platelets for room temperature X-ray detection. IEEE Transactions on Nuclear ... Given the phase diagram for iodine (s) below, predict what happens when an iodine sample is heated from 50°C to 250°C at a constant pressure of 1 Question : For the phase diagram given, match the labels for: lodine, 12 D Pala) с A 2 . 30 100 150 fo 500 1260) Regions A, B, C and D Lines 1, 2 and 3 Points a and b A Choose... Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius, and boils to a violet gas at 184 degrees Celsius. Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
The melting point-composition diagram for the thallium-iodine system has been determined. There are three compounds: TlI, Tl 3 I 4, and TlI 3. From Tl to TlI the solids and liquids are immiscible. From TlI to I 2 there is one liquid phase. Solid Tl 3 I 4 melts incongruently at 260 °C to a liquid (atom fraction iodine = 0.77) and solid TlI. Phase equilibriums are studied in the isothermal-isobaric sections of the phase diagram of a fourcomponent iodine-potassium iodide-water-ethanol system at 25°C and atmospheric pressure. The compositions of the solvent at which it exhibits the greatest ability to dissolve iodine are established. Chemistry questions and answers. Question 1 (1 point) Given the phase diagram for iodine (below) match the region, line and point names as labelled on the diagram (note - the choices are a bit awkward - you have to select the number that corresponds to your choice - you don't input the choice directly.) lodine, 12 Plate) b c fo OL 2 a OOK B 70 ... The INFO file contains approximately 3,000 entries of literature references that contain phase equilibrium information but, in general, no diagram (or a diagram that does not obey the phase rule). These papers have been retrieved from the archival literature during our systematic searches for new content for the database.
Answer to: Consider the phase diagram for iodine shown below and answer each of the following questions (Figure 1) What phase is present at 186 degrees C and
The phase diagram of iodine is as follows: Iodine consists of 12 molecules and the attraction between its molecules is only the Van der Waals... See full answer below. Become a member and unlock...
Problem: Consider the phase diagram for iodine shown at the top of the next page.a. What is the normal boiling point for iodine?b. What is the melting point ...
In addition, the Help menu provides a downloadable .pdf file of the classic tutorial General Discussion of Phase Diagrams, by F.P. Hall et al. (containing a glossary of phase-equilibrium terminology and thermodynamic definitions), background information for using the database (sections Common Tasks, FAQ, Chemical System Designation Rules), and a detailed history of this project and its ...
Thus, the notion that upon heating iodine only sublimes, ... Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, ...
Download scientific diagram | Phase diagram of iodine (retrieved from http://chemwiki.ucdavis.edu/Textbook). from publication: A Demonstration of the Sublimation Process and its Effect on Students’ Conceptual Understanding of the Sublimation Concept | In this paper, we present a demonstration for ...
Iodine | I2 | CID 807 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ...
For a better comparability, the two phase region is separated by horizontal dotted lines. In the two phase region (0.29 (±0.02) ≤ x ≤ 0.92 (±0.02)) the iodine rich phase is increasingly incorporating bromine, over the whole composition range up to 80%. Simultaneously, however, the fraction of this phase decreases.
Draw the phase diagram for iodine labelling the different phases. What are the nature of the intermolecular interactions that exist between iodine molecules? Answer +20. Watch. 1. answer. 1. watching. 171. views. For unlimited access to Homework Help, a Homework+ subscription is required.
(Please check your downloads folder shortly for your download) · If you have a problem obtaining your download, click here to go back to the article page. Or contact our support team who will be happy to help
Therefore, for the given phase diagram: The region found at 1 atm and 186°C is the gaseous phase region. What state is iodine at room temperature and atmospheric pressure? The state of iodine at room temperature and normal atmospheric pressure is solid. Part D. Based on the graph, The state of iodine at 186 °C and 1.0 atm is gas.
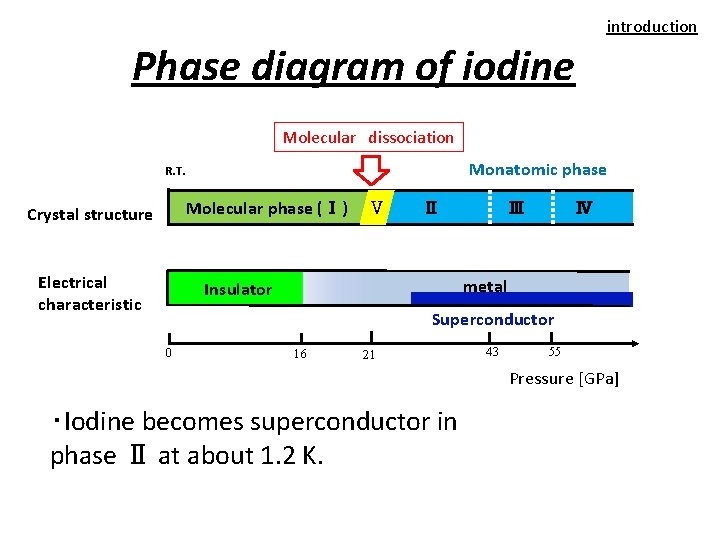
molecular phase at low pressures, even before the onset of phase V. Here, we suggest a phase for solid iodine in such a low-pressure range (designated as phase I) in which two different covalent intramolecular bonds coexist, based on first-principles calculations and later corroborated by x-ray diffraction experiments. The pres-
March 24, 2017 - The triple point of Iodine occurs at 0.12 atm and at standard temperature it is a solid on the phase diagram. I understand that therefore, when heating iodine at 1 atm, it (at least some of it) will
OSTI.GOV Journal Article: On the phase diagram of mercuric iodide near the stoichiometric composition
Chemistry questions and answers. Consider the phase diagram for iodine shown below and answer each of the following questions (Figure 1). Figure < 1 of 1 m Pressure (not to scale) 113.6°C 184.4°C TC Temperature (not to scale) Part A What is the normal boiling point for iodine? Express your answer in degrees Celsius using four significant figures.
March 3, 2021 - Elemental iodine, I2, forms dark gray crystals that have an almost metallic appearance. It is often used in chemistry classes as an example of a solid that is easily sublimed; if you have seen such a demonstration or experimented with it in the lab, its phase diagram might be of interest.
Question: Consider the phase diagram for iodine shown here. a. What is the normal boiling point for iodine? b. What is the melting point for iodine at 1 atm? c. What state is present at room temperature and normal atmospheric pressure d. What state is present at 186 °C and 1.0 atm? Pc 1 atm Pressure (not to scale) 113.69184.4 CMZO Temperature ...
The triple point of iodine is at 110 ° C and 0.12 atm and the critical point of iodine is at 335 ° C and 116 atm. Q 1. Based on the phase diagram above, which statements are correct? 1 Sublimation occurs at a point in the transformation along a straight line from point A to F. 2 C and E represent points where the gas and liquid phases are in ...
Phase diagram of iodine. A phase diagram describes the situation in which one is observing: a single chemical substance, in a closed system, under equilibrium conditions. Mike's experiment involves not a single substance (it also contains air), is an open system, and is not at uniform (equilibrium) temperature.
Phase diagram of iodine Elemental iodine, I 2 , forms dark gray crystals that have an almost metallic appearance. It is often used in chemistry classes as an example of a solid that is easily sublimed; if you have seen such a demonstration or experimented with it in the lab, its phase diagram might be of interest.
Phase equilibriums are studied in the isothermal-isobaric sections of the phase diagram of a fourcomponent iodine-potassium iodide-water-ethanol system at 25°C and atmospheric pressure. The compositions of the solvent at which it exhibits the greatest ability to dissolve iodine are established.
August 2, 2016 - Something went wrong. Wait a moment and try again
Solution for iClicker Question- To the right is the phase diagram for iodine. Which of the following transitions occur when the temperature and pressure…
Consider the phase diagram for iodine shown below and answer each of the following questions. What is the melting point for iodine at 1 atm? Learn this topic by watching Phase Diagrams Concept Videos All Chemistry Practice Problems Phase Diagrams Practice Problems Q. ...
Question: Part A What is the normal boiling point for iodine? Part B What is the melting point for iodine at 1 atm? Part C What phase is present at room temperature and normal atmospheric pressure? Part D What phase is present at 186 ?C and 1.0 atm?
Phase diagram of iodine (not to scale): 1 = triple point, 2 = normal melting point, 3 = normal boiling point. Re-elaborated from literature numerical values. 2. The experimental setup. At the ...
May 14, 2015 - Phase equilibria in the cross sections of isothermal-isobaric sections of the phase diagram of four-component iodine-potassium iodide-water-propyl alcohol are investigated at 298.15 K and pressure of 101325 Pa. It is shown that a three-phase equilibrium of the eutonic type occurs in the cross ...
Access 130+ million publications and connect with 20+ million researchers. Join for free and gain visibility by uploading your research.
(a) The temperature of a solid sample is held at 113.5°C while heat is added.: Solid iodine, I 2, melts to form liquid I 2. (b) The temperature of a sample is lowered from 452 K to 389 K. These temperatures are ca. 179°C and 116°C, respectively.
Iodine. Formula: I 2. Molecular weight: 253.80894. IUPAC Standard InChI: InChI=1S/I2/c1-2. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: PNDPGZBMCMUPRI-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet. CAS Registry Number: 7553-56-2. Chemical structure:
Video explaining Phase diagram iodine for General Chemistry. This is one of many Chemistry videos provided by ProPrep to prepare you to succeed in your university
April 2, 1999 - Pressure–temperature diagrams of selenium, sulfur and iodine melts are discussed in connection with the experimental data on volumetric and resistance anomalies in the liquid state. The problem of 1st order phase transitions in the liquid state is briefly discussed.
Answer: 7.5: Changes of State Scroll down to Figure 7.5.14: Phase diagram of I2
September 1, 2012 - Phase equilibriums are studied in the isothermal-isobaric sections of the phase diagram of a fourcomponent iodine-potassium iodide-water-ethanol system at 25°C and atmospheric pressure. The compositions of the solvent at which it exhibits the greatest ability to dissolve iodine are established.
Consider the phase diagram for iodine shown here and answer each of the following questions. a. What is the normal boiling point for iodine? b. What is the melting point for iodine at 1 atm? c. What state is present at room temperature and normal atmospheric pressure? d. What state is present at $186{ }^{\circ} \mathrm{C}$ and $1.0 \mathrm{~atm}$ ?
Inconsistent explanations, then, can serve as a basis for developing misconceptions and preconceptions in latter students' education. Thus, the notion that upon heating iodine only sublimes, but does not melt is present in many chemistry textbooks, teachers lectures and, therefore, in students ...







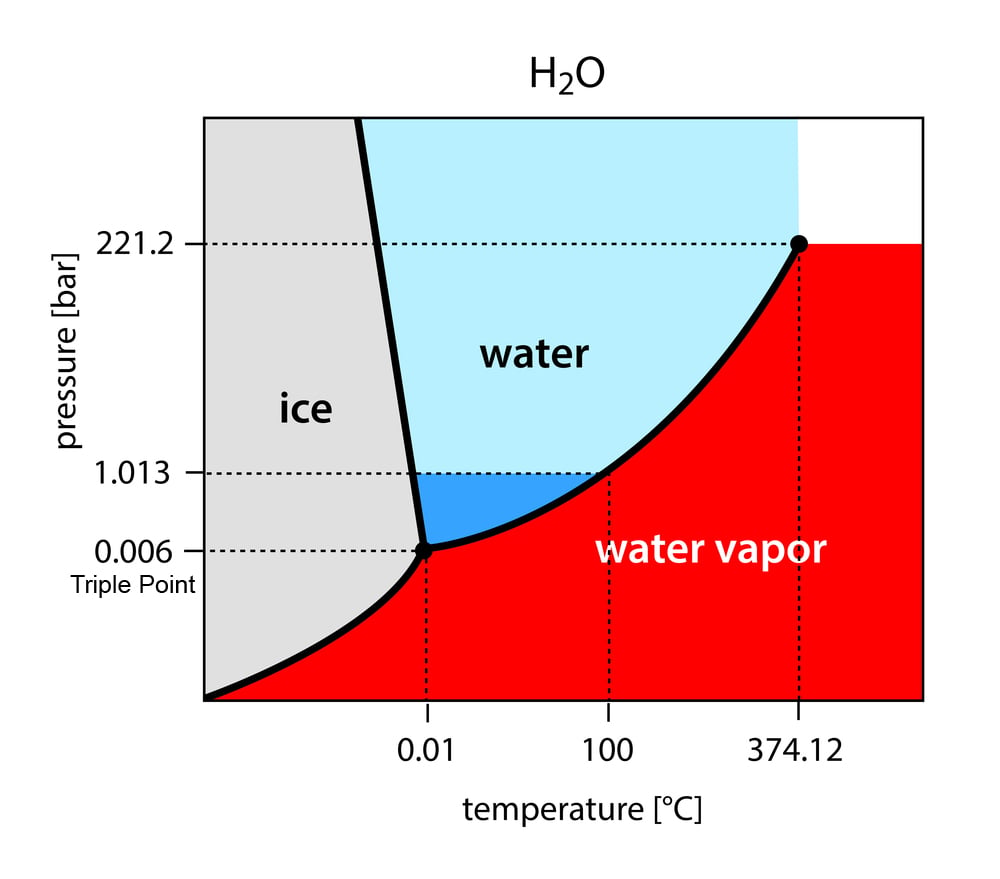







![A Dual Plating Battery with the Iodine/[ZnIx(OH2)4−x]2−x ...](https://onlinelibrary.wiley.com/cms/asset/6f0a57ae-4f40-4d10-a379-840b00e964a1/anie201909324-fig-0001-m.jpg)







0 Response to "41 phase diagram for iodine"
Post a Comment