38 endothermic reaction energy diagram
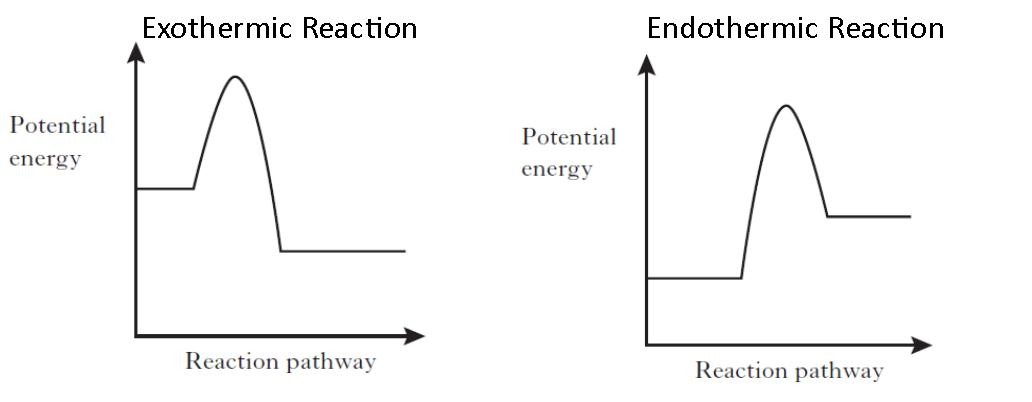
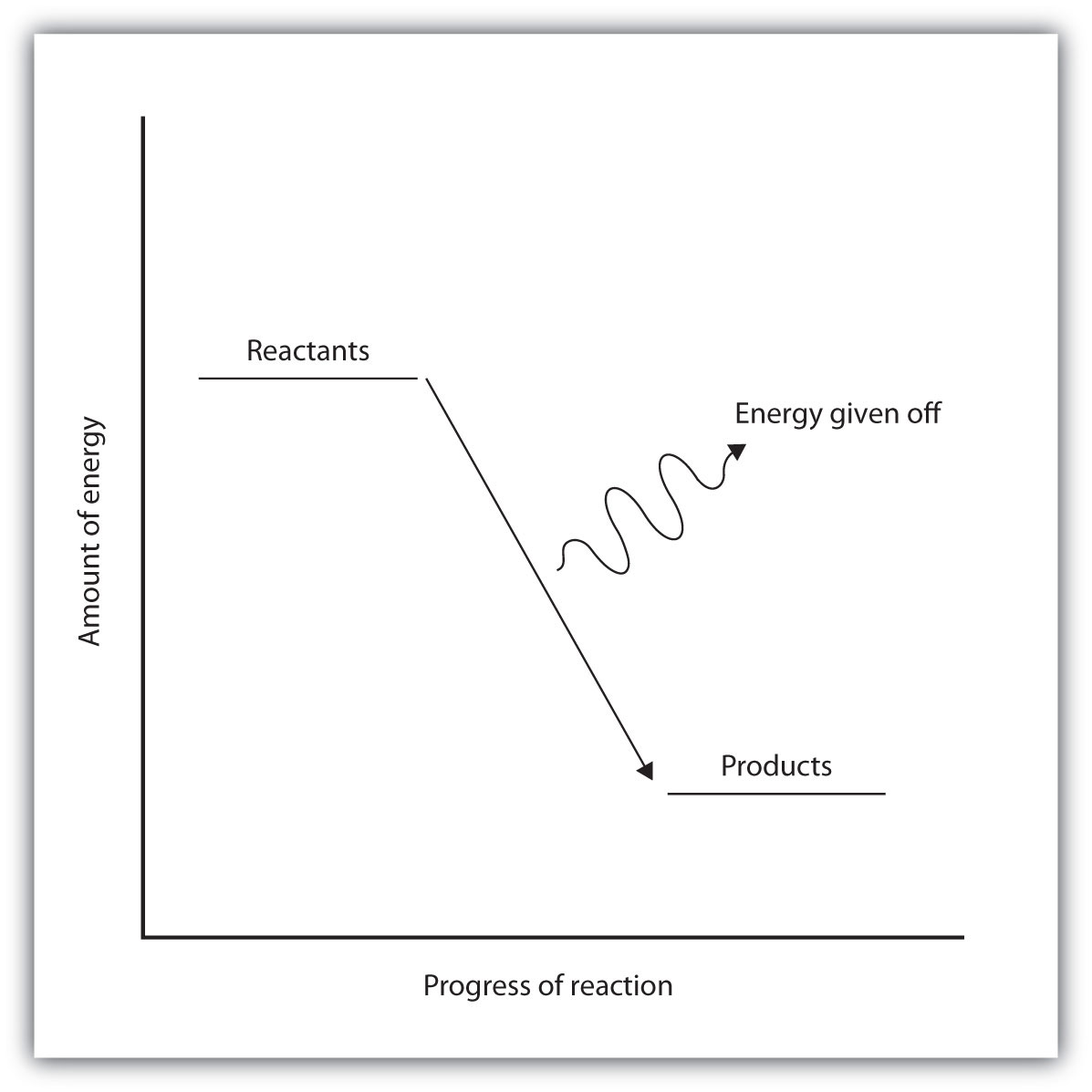
The Cold Pack: A Chilly Example of an Endothermic Reaction ... Jun 01, 2020 · Energy diagrams show the energy levels of reactants and products in a reaction. General energy diagrams for exothermic and endothermic reactions (©2020 Let’s Talk Science). You can see from the diagram above that the energy level of the products of an exothermic reaction is lower than the energy level of the reactants. Endothermic vs. exothermic reactions (article) | Khan Academy Endothermic vs. exothermic reactions · This is the currently selected item. Test prep·MCAT·Chemical processes·Thermochemistry ... Endothermic vs. exothermic reactions
Topic 5.1 Exothermic and Endothermic Reactions Heat Kindergarten Registration for the 2022-2023 school year will once again occur online through Infinite Campus (our student information system). The online system will walk parents/guardians through the registration process and provide links to upload required documentation.

Endothermic reaction energy diagram
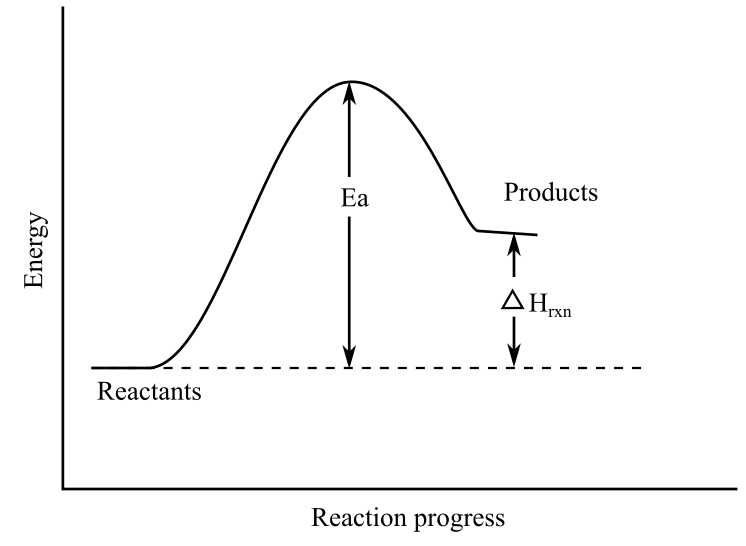
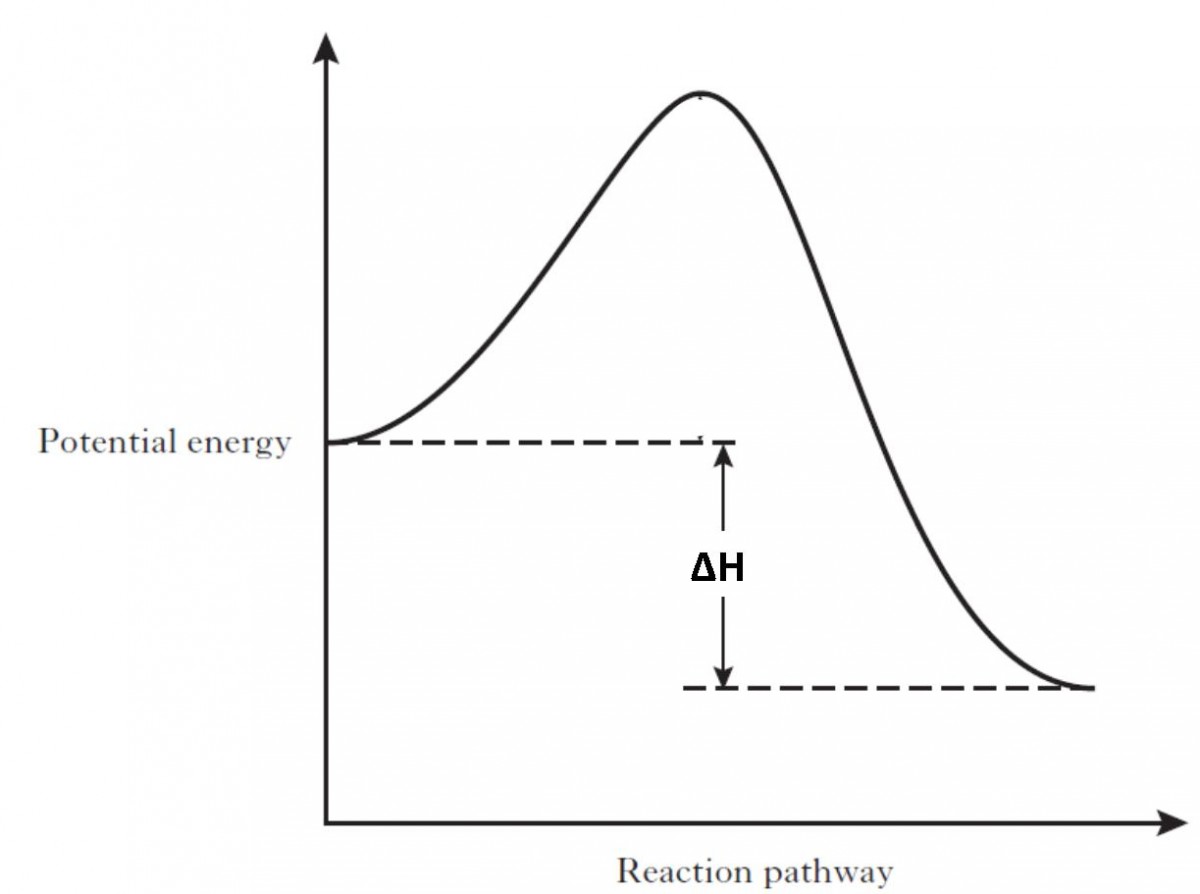
Energy Diagrams | OpenOChem Learn Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0). How can I represent an endothermic reaction in a potential energy ... December 20, 2015 - An endothermic reaction must have a positive change in enthalpy. That is, Delta"H">0. This means that the system absorbs heat. To represent the positive change in enthalpy, the potential energy of the reactants must be LOWER than that of the products. Notice how the end of the "hump" is higher ... Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ...
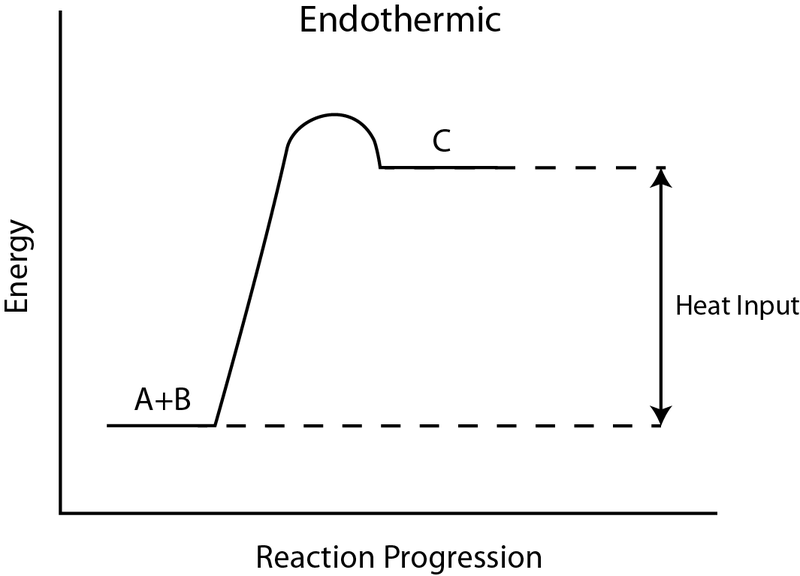
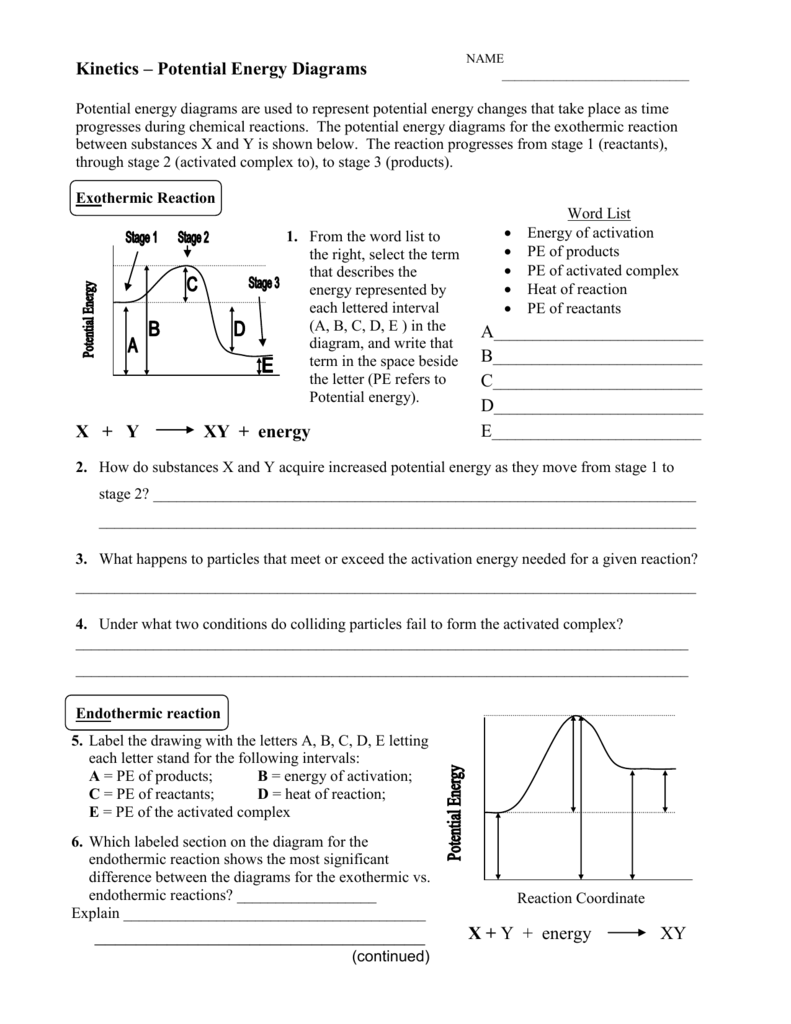
Endothermic reaction energy diagram. How do you know if a diagram is endothermic or exothermic ... In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0). By examining this enthalpy change, one can tell whether a reaction is endothermic (ΔH>0) or exothermic (ΔH<0). PDF Exothermic vs endothermic reaction graphs State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer-- Potential Energy Diagrams - Kentchemistry.com 43 Given the potential energy diagram for a chemical reaction: Which statement correctly describes the energy changes that occur in the forward reaction? (1) The activation energy is 10. kJ and the reaction is endothermic. (2) The activation energy is 10. kJ and the reaction is exothermic. How does the energy level diagram show this reaction is ... In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:
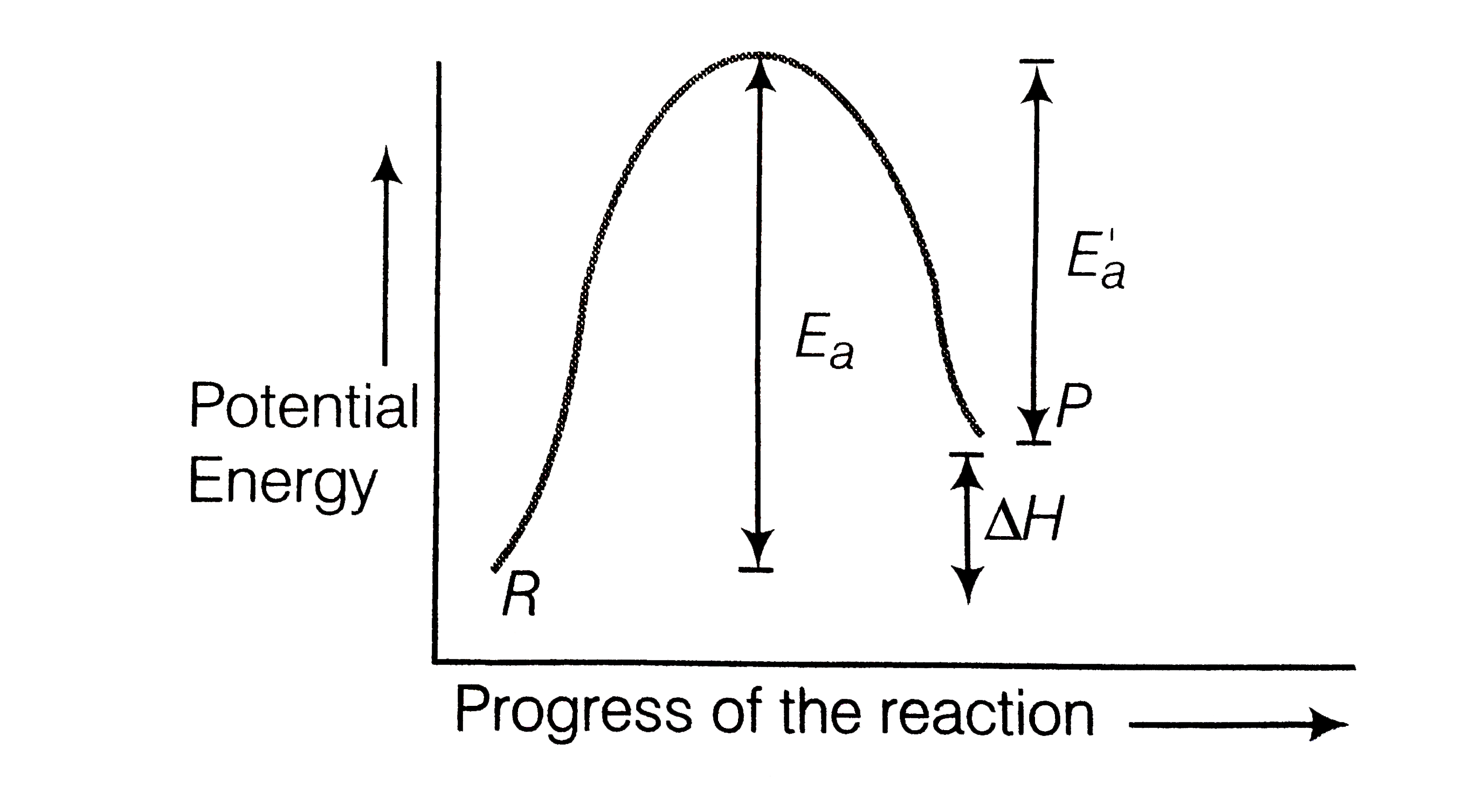
Potential Energy Diagrams (3) The reaction is endothermic because it releases heat. (4) The reaction is endothermic because it absorbs heat. 46 Given the potential energy diagram for a reaction: Endothermic Reaction Coordinate Diagram - Wiring Diagrams A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other. Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants and ΔH is ... PPTX Energy and Reactions Energy level diagram for an exothermic chemical reaction without showing the activation energy.It could also be seen as quite exothermic with a highly unlikely zero activation energy, but reactions between two ions of opposite charge usually has a very low activation energy. Could usually . has a very low activation energy.
Endothermic vs. exothermic reactions (article) | Khan ... Endothermic reactions: Heat is absorbed. 1) Photosynthesis: Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. 6CO2 + 6 H2O + heat ---> C6H12O6 + 6O2. 2) Cooking an egg: Heat energy is absorbed from the pan to cook the egg. PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. PDF Potential Energy Diagrams ENDOTHERMIC OR EXOTHERMIC? Note the REACTANTS and PRODUCTS are labeled. The reactants are higher in energy (higher on y axis) then products, indicating energy is released (lost) as chemical reaction occurs, so this is an exothermic reaction. ΔH is the total energy change (enthalpy) of the reaction. ΔH is the difference in energy between the What are Endothermic Reactions? (with Examples & Video) Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ...
Reaction profiles - GCSE Chemistry (Single Science) Revision An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
18.4: Potential Energy Diagrams - Chemistry LibreTexts 1 month ago - The energy changes that occur during ... called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an ...
Reaction Coordinate Diagrams The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
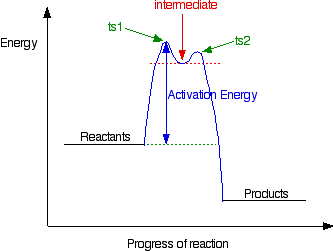
Energy Profiles for Simple Reactions - Chemguide A closer look at energy profiles for reactions involving either a transition state or an intermediate
Bond Energies - Chemistry LibreTexts Aug 21, 2020 · Total energy difference is 1840 kJ/mol – 1371.5 kJ/mol = 469 kJ/mol, which indicates that the reaction is endothermic and that 469 kJ of heat is needed to be supplied to carry out this reaction. This reaction is endothermic since it requires energy in order to create bonds.
Creative Diagram Of Exothermic Reaction - Glaucoma Template Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure PageIndex2. Based on Figure the following information can be obtained. An exothermic reaction is a reaction in which energy is released in the form of light or heat.
Exothermic and Endothermic Processes | Introduction to ... Endothermic and exothermic reactions Paul Andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically.
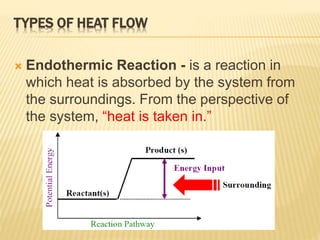
Endothermic Reaction | Characteristics, Examples ... An Endothermic reaction is a chemical change in which the System absorbs thermal energy from its Surroundings resulting in the overall increase in its total internal energy level or Enthalpy. The ...
Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
Representing endothermic and exothermic processes using energy ... October 5, 2021 - A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. If the initial state has a lower potential energy than the final state, the process is endothermic.
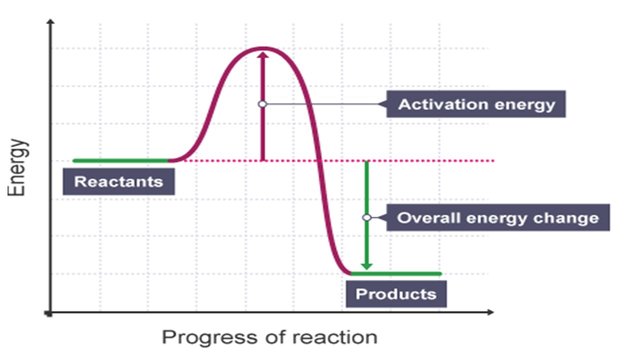
Activation energy - Why are there energy changes in chemical ... The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction.
12.3 Activation energy and the activated complex | Energy and ... Recall from earlier that we drew graphs for the energy changes in exothermic and endothermic reactions. We can now add some information to these graphs. This will also explain why we draw these graphs with a curve rather than using a straight line from the reactants energy to the products energy.
Gibbs Free Energy - Purdue University Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.
6.3.1 This page examines simple energy profiles for reactions, and shows how they are slightly different for reactions involving an intermediate, as opposed to just a transition state.
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Reaction Coordinate Diagram Endothermic Vs Exothermic Energy diagrams for endothermic and exothermic reactions. This is shown in an Energy vs Reaction Coordinate plot. useful in showing: 1) whether a reaction is an exo- or endothermic process, 2) which reaction Exothermic reactions will have products that are lower in energy than the reactants.Endothermic Reaction Energy Diagram - 62 Unique Figure ...
Energy changes - Energy changes in chemical reactions - GCSE ... An energy level diagram for an endothermic reaction Activation energy The activation energy is the minimum amount of energy required for a reaction to take place.
Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... Label your diagram - if you are filling in electronically, click on diagram, click "edit" and fill in correct words in text boxes already provided. Key things to Note about EXOTHERMIC Energy Diagram: Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction (this is why products have less energy) Δ H will be negative Task 3 ...
Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... Task 2: Introduction to Energy Diagrams Click here to fill help you fill in this task: Endothermic vs. Exothermic and Energy Diagrams Reference Slides Energy Diagram Shows the changes in potential energy during a reaction. It starts with the _reactants_____ on the left and proceeds to the ____products_____ on the right.
Solved The reaction energy diagrams for an endothermic and ... The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of ...
18.4: Potential Energy Diagrams - Chemistry LibreTexts 21 Feb 2022 — The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy ...
Representing endothermic and exothermic processes using ... In the profile for an exothermic reaction, the overall change is negative. You can tell this because the products have less energy than the reactants, and the arrow showing the overall change in energy points downwards. The diagram shows a reaction profile for an endothermic reaction.
Energy Diagram — Overview & Parts - Expii sketch the reaction energy diagram for a single-step reaction, given some indication of whether the reaction is fast or slow, exothermic or endothermic. interpret the reaction energy diagram for a single-step process (e.g., use the diagram to decide whether the reaction is exothermic or ...
PDF Exhothermic and Endothermic Reactions / Activation Energy ... Page 2 of "Activation Energy / Exhothermic and Endothermic Reactions" • An ENDOTHERMIC Process i.e. one where ∑ H for the Products > ∑ H for the Reactants (therefore ∆H is positive). NB ∆H is the amount of energy absorbed/consumed in the reaction and it is given by ∑ H for the Products minus ∑ H for the Reactants.
Draw an energy diagram for an endothermic reaction without ... Explanation: Endothermic reactions are defined as the reactions in which energy of the product is greater than the energy of the reactants.The total energy is absorbed in the form of heat and for the reaction comes out to be positive.. Catalyst is the substance which does not participate in the reaction but increases the rate of the reaction and results in the early formation of product.
Examples of Chemical Energy in Everyday LIfe 9 Examples of Chemical Energy. Chemical energy is either released (exothermic reaction) or absorbed (endothermic reaction) during a chemical reaction. In an exothermic reaction, heat is released, creating warmth. In an endothermic reaction, the heat is absorbed, creating cooling. Air bags are activated by a chemical reaction inside the bag. A ...
Energy Profiles (Energy Diagrams) Chemistry Tutorial An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change , ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products.
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ...
How can I represent an endothermic reaction in a potential energy ... December 20, 2015 - An endothermic reaction must have a positive change in enthalpy. That is, Delta"H">0. This means that the system absorbs heat. To represent the positive change in enthalpy, the potential energy of the reactants must be LOWER than that of the products. Notice how the end of the "hump" is higher ...
Energy Diagrams | OpenOChem Learn Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0).






















0 Response to "38 endothermic reaction energy diagram"
Post a Comment