39 lewis dot diagram for o3
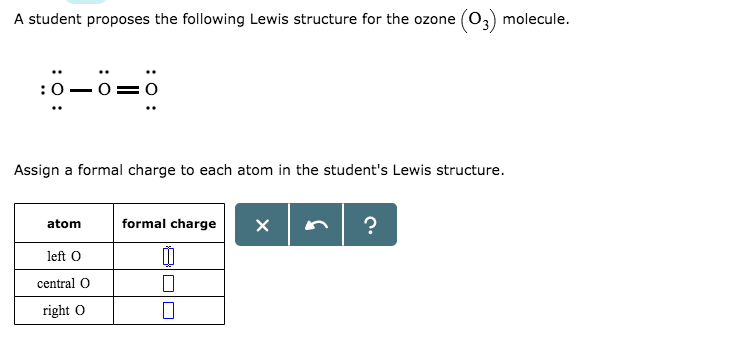
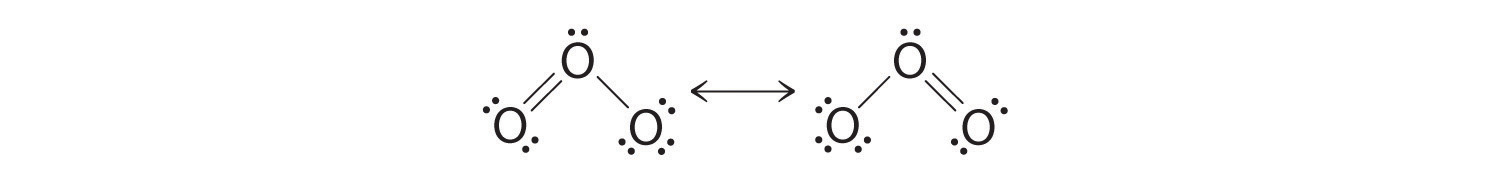
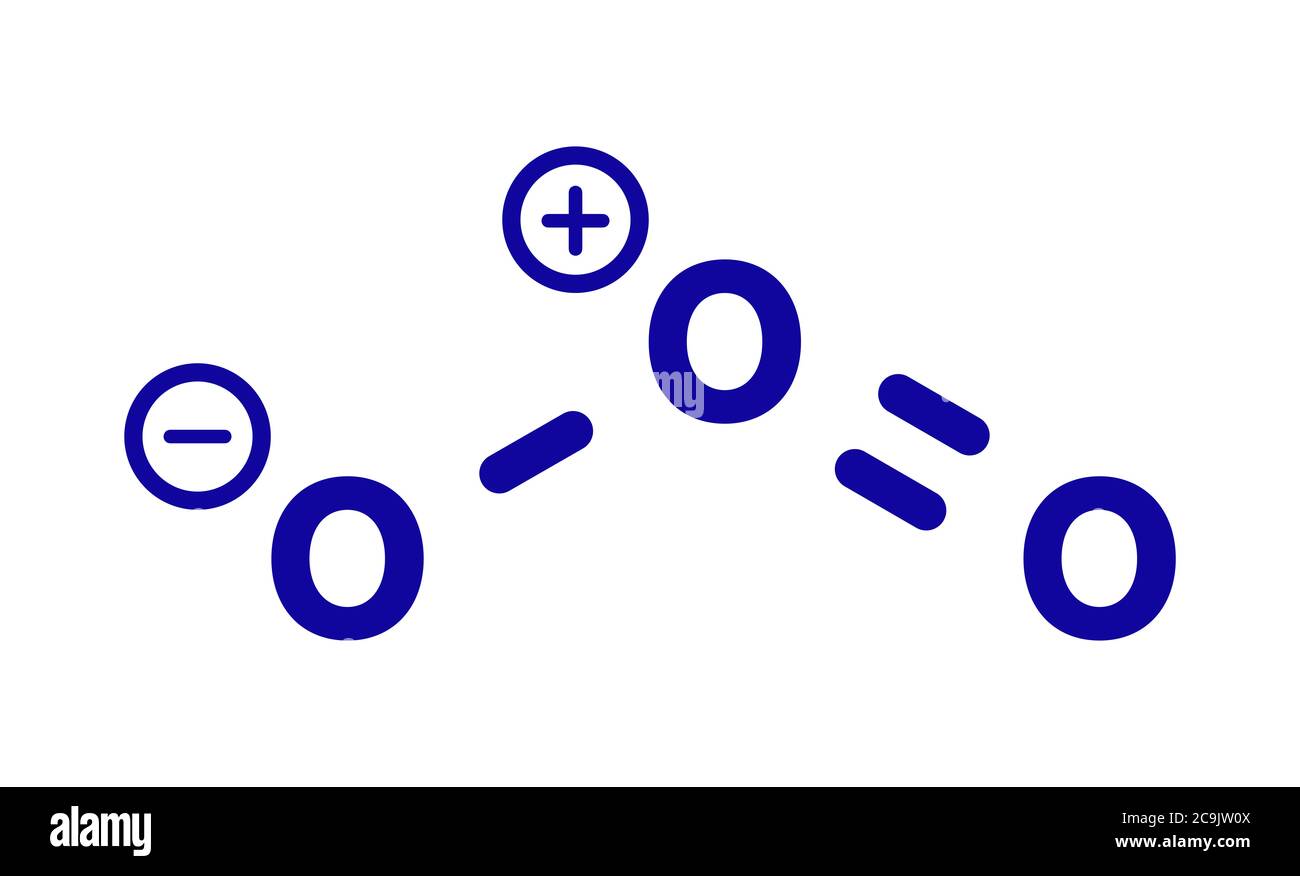
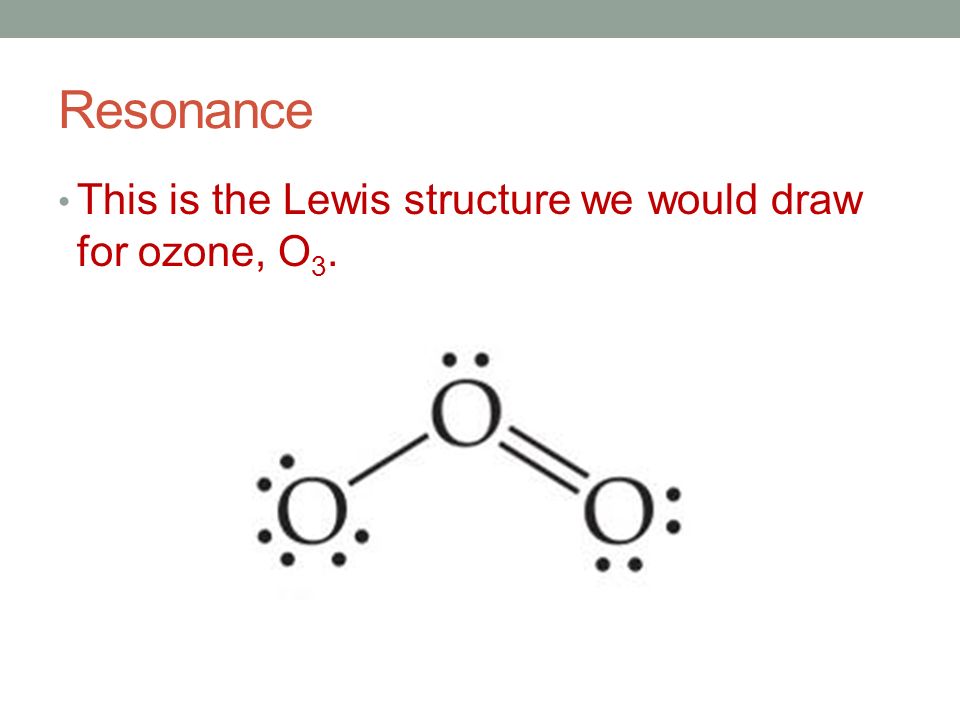
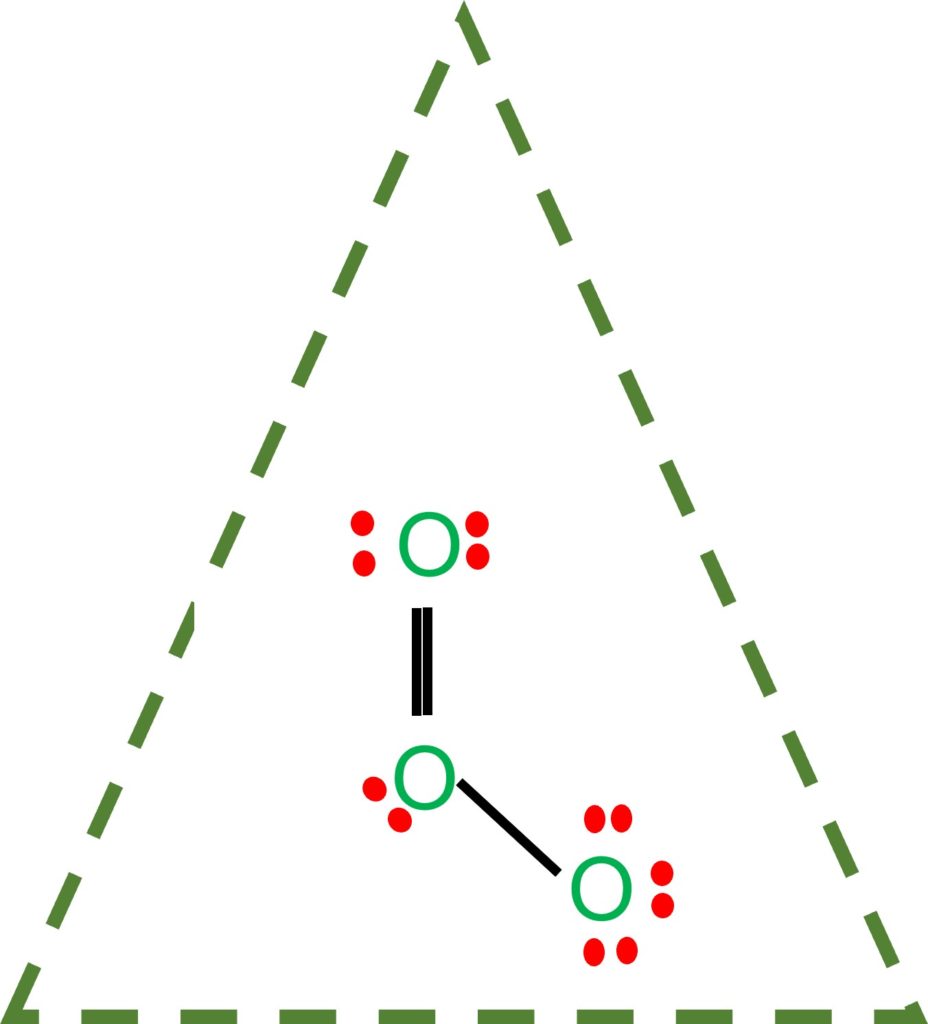
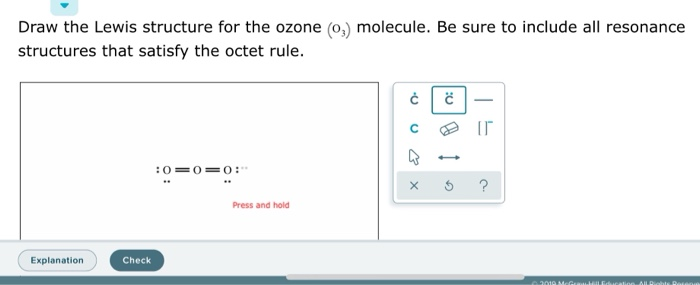
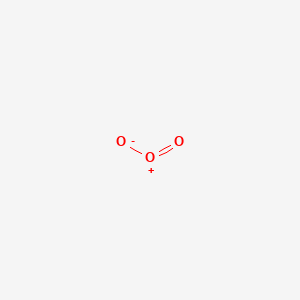
Draw the lewis structure for: SiO3 -2 CNO- TeO4 -2 F2PPCl2 ... Lewis Dot structure bond length molecular geometry molecular formula Molecular geometry? chemistry. C4 H10 O or diethyl ether or with a formula of CH3CH2OCH2CH3 its stick structure is H H H H ! ! ! ! .. H-C-C-C-C- O-H ! ! ! ! .. H H H H I used the this ! sign as a sign for bond. pls help me to determine the geometry for each central atom in this What is the Lewis structure of ozone (O_3)? | Socratic Explanation: Simple VESPER requires that we distribute 3 ×6 = 18 valence electrons across 3 centres: O = O+ − O−. From the left, O1, has TWO lone pairs; O2 has ONE lone pairs; and O3 has THREE lone pairs. And thus the formal charge of each oxygen atom ( 8e−,7e−,9e−) is 0, + 1, −1 respectively. Because there are THREE regions of ...
Lewis Dot Structure of O3 (Ozone) - YouTube I quickly take you through how to draw the Lewis Structure of O3 (Ozone). I also go over the resonance, hybridization, shape and bond angle.

Lewis dot diagram for o3
CO2 Lewis Structure | Lewis Dot Structure For Molecules ... Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure works on the octet rule, which means that all the atoms would have eight electrons in their valence shell except hydrogen. Lewis Dot Diagram For O3 - schematron.org I was wondering why the Lewis structure for O3 is O-O=O ( where the Formal charge from left to right is -1,+1,0) (3 e- pairs. Consider the case of ozone O3 Lewis electron dot structures: 2 π electrons (pi electrons) in O3 and so 1 double bond must be added to the structure of Step 1.How to Draw a Lewis Structure Find the Total Number of ... O3 Lewis Structure - How to Draw the Dot Structure for O3 ... A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence ...
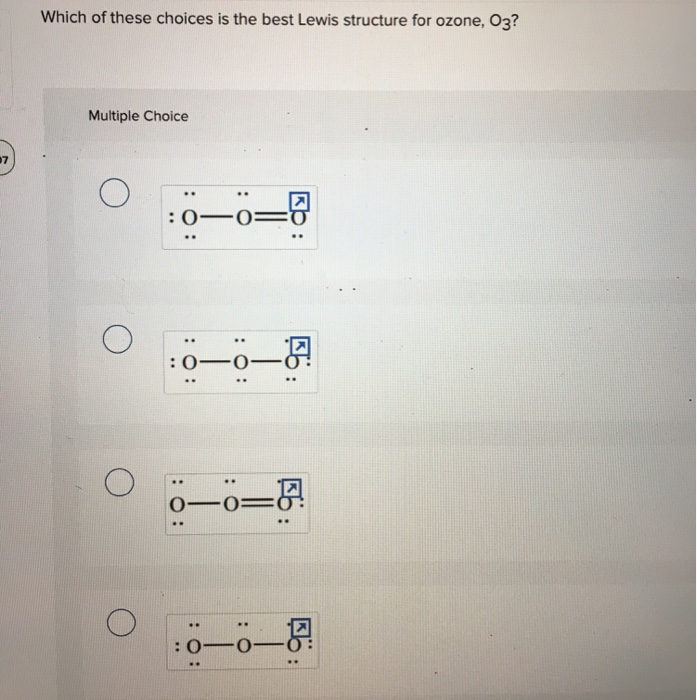
Lewis dot diagram for o3. Lewis dot structure for O3? - Answers Lewis dot structure for O3. Wiki User. ∙ 2010-12-06 00:29:36. Study now. See answer (1) Best Answer. Copy..:O=O: |:O:.. this would have a resonance structure too. 18 electrons total. 1 single ... Lewis Dot Structure For Oxygen - Smart Drawing the Lewis Structure for O 2. So according to the lewis dot structure of OF2 oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons. For example oxygen has 6 valence electrons so we write the symbol O for oxygen and surround it with 6 dots. Lewis Dot Structure e Flashcards - Quizlet Draw the Lewis Dot Structure for O3? What is the total number of valence ELECTRONS? 18 valence electrons. Draw the Lewis Dot Structure for SO3^2-? What is the total number of valence ELECTRONS? 26 valence electrons. YOU MIGHT ALSO LIKE... 133 terms. Lewis Dots & Molecular Bonding. 66 terms. What is the Lewis structure for ozone? - Quora The Lewis structure of ozone (O3) 1. Sum of valence electrons = (6*3) = 18 2. Drawing the bond connectivities: 3. Complete the octets of the atoms bonded ...
O3 Resonance Structures - How to draw the resonance ... But again, when we draw those resonance structures, it's to kind of get around a limitation with how we have these rules to draw Lewis structures. This is really what the molecule would look like in the real world. This is Dr. B. with the resonance structure for O3, ozone. Thanks for watching. Lewis Dot of Ozone O3 - kentchemistry.com Lewis Dot of Ozone. O 3. Back. 70 More Lewis Dot Structures. Element number 8 and a member of the Chalcogen Family or Group 16 of the periodic table. Ozone is an allotrope of oxygen, and is much less stable. Ultraviolet light cause it to decompose in our ozone layer, therefore it shielding people below it. How To Draw Lewis Structure For O3 Ozone - Dubai Burj Khalifas O3 Lewis Structure How To Draw The Dot Structure For O3. a step by step explanation of how to draw the o3 lewis dot structure (ozone). for the o3 structure use the periodic table to find the lewis structure of ozone has: * three oxygen atoms in a row. it is not a ring, although that might be tempting. * two resonance how to draw lewis structure for o3 ozone lewis structure: this chemistry ... Lewis Structure For Ozone - o3 lewis structure ozone ... Lewis Structure For Ozone - 9 images - lewis electron dot structures ck 12 foundation, computational chemistry energetic materials lmu munich, ... O3 Resonance. Draw All Resonance Structures For The Ozone Molecule O3. O2 2 Lewis Structure. Oxygen Lewis Dot Diagram.
PDF Lewis Dot Structures and VSEPR - Surry County Public ... • (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. O3 Lewis Structure, Molecular Geometry, Hybridization, and ... Lewis Structure of O3. Here, we will be dealing with ozone, the molecular formula is O3. The below discussion, therefore, will be based on finding out the Lewis Structure of O3. Ozone consists of three oxygen atoms. Oxygen belongs to group VI of the periodic table with an atomic no of 8. It thus has 6 valence electrons. Ozone | O3 - PubChem Ozone is an elemental molecule with formula O3. An explosive, pale blue gas (b.p. -112℃) that has a characteristic, pleasant odour, it is continuously produced in the upper atmosphere by the action of solar ultraviolet radiation on atmospheric oxygen.It is an antimicrobial agent used in the production of bottled water, as well as in the treatment of meat, poultry and other foodstuffs. What is the Lewis dot structure of O3? - Answers O3 is called ozone and it looks like this:O = O - OThe dots go around the oxygens in order of how many more they need to make 8.The other answer for this isO - O = Oand then fill in the other ...
O3 Lewis Structure, Polarity, Hybridization, Shape and ... The central atom in the Lewis structure will have a charge of +1 and the atom forming a single bond will have -1 charge. O3 Hybridization Hybridization in chemistry means the hybridising of two or more atomic levels of the same or different energies to combine and give a new orbital.
Al2O3 Lewis Structure, Molecular Geometry, Hybridization ... Lewis Structure of Al2O3 The concept of Lewis structure was first introduced by Gilbert N. Lewis in 1916. It is also known as the Lewis dot diagram or electron dot structure. It is the structural illustration of the position of the valence electrons, involved in the formation of a chemical bond, around the atoms inside a molecule.
O3 Lewis Structure: How to Draw the Dot Structure for O3 ... For the Lewis Structure for O 3 it is possible to draw it two different says (slightly different, but still important). These are called resonance structures. O 3 (Ozone) is important in the upper atomsphere since it blocks UV light that can be harmful to humans (for example: causing skin cancer).
What Is The Lewis Dot Structure For ClO3-? - Answers ~ The ... Lewis Dot Structure of ClO3- (Chlorate Ion).Steps of drawing ClO 3- lewis structure. Find total number of electrons of the valance shells of chlorine and oxygen atoms and including charge of the anion.A step-by-step explanation of how to draw the ClO3- Lewis Structure (Chlorate Ion). The ClO3- Lewis structure is a good structure to help you ...
Ozone (O3) Lewis Structure - Steps of Drawing Ozone (O3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double bond and one single bond. Also, there are charges in two oxygen atoms in O 3 lewis structure. Lewis structure of O 3 can be drawn by starting from valence electrons of oxygen atoms in several steps.
Lewis Dot O3 - Novocom.top lewis o3 dot diagram structure ozone chem oxygen draw hi possible study guide atwood 1100 number bond allotrope . lewis dot o3 diagram structures . lewis structure o3 ozone dot formal stack slidesharefile charges chemistry exchange . oxygen o3 examples allotropes formulas lewis structure ozone study .
Simple Method for writing Lewis Structures for N2O3 ... Lewis Electron Dot Structures - Simple Procedure for writing Lewis Structures - Lewis Structures for Dinitrogen Trioxide (N2O3), n2o3 lewis structure, how to write a lewis structure, N2O3, lewis electron dot structure of N2O3, σ bonds in N2O3, π bonds in N2O3, calculate the number of electrons in π bonds, resonance structures in N2O3, octet rule in n2o3, Lewis structures for dinitrogen ...
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Simple method for writing Lewis Structures - Ozone O3 and ... what is the Lewis electron dot structures for ozone O3, pi and, what is the Lewis electron dot structures for carbonates CO3-2, Simple Procedure for writing Lewis Structures for O3 and carbonate ion, co32-, o3 lewis structure, lewis structure, lewis dot structure, CO3-2 lewis structure, O3 lewis structure, lewis dot diagram, electron dot diagram, Chemistry Net, chemistry tutorial on Lewis ...
O3 Lewis Structure - How to Draw the Dot Structure for O3 ... A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence ...
Lewis Dot Diagram For O3 - schematron.org I was wondering why the Lewis structure for O3 is O-O=O ( where the Formal charge from left to right is -1,+1,0) (3 e- pairs. Consider the case of ozone O3 Lewis electron dot structures: 2 π electrons (pi electrons) in O3 and so 1 double bond must be added to the structure of Step 1.How to Draw a Lewis Structure Find the Total Number of ...
CO2 Lewis Structure | Lewis Dot Structure For Molecules ... Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure works on the octet rule, which means that all the atoms would have eight electrons in their valence shell except hydrogen.







![High School Chem]: Is this the correct Lewis Structure for ...](https://preview.redd.it/pzmn9pdp2io41.jpg?auto=webp&s=9778348f6a62c80dcdd0eb84a10bbcb32da471bf)



![Expert Answer] Calculate the formal charge of each oxygen ...](https://hi-static.z-dn.net/files/d09/adf07c543450893ab4a33b837e9579c7.png)








0 Response to "39 lewis dot diagram for o3"
Post a Comment