39 liquid vapor phase diagram
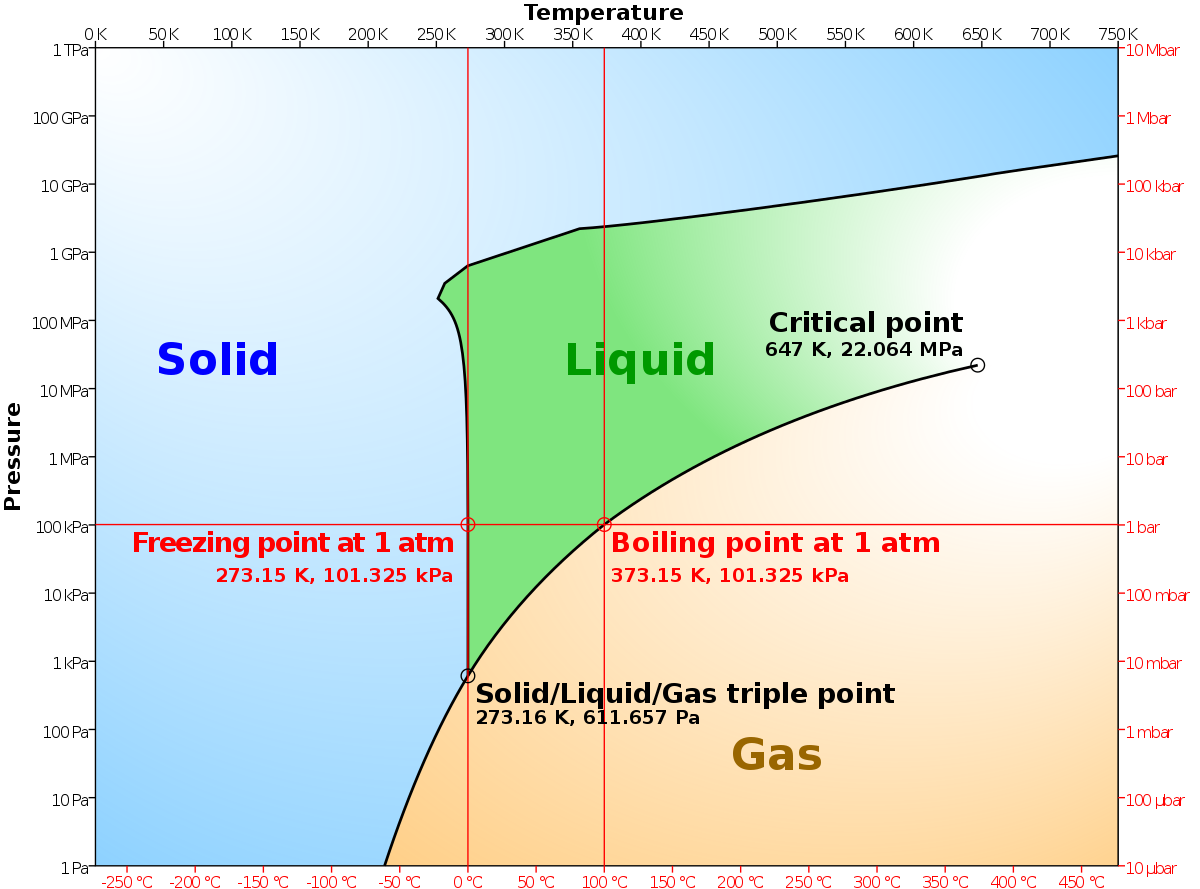
Phase Diagrams - Chemistry - University of Hawaiʻi This "liquid-vapor" curve separates the liquid and gaseous regions of the phase diagram and provides the boiling point for water at any pressure. For example, at 1 atm, the boiling point is 100 °C. 39 solid liquid phase diagram - Wiring Diagrams Manual PDF Liquid-Solid Phase Diagrams - Texas A&M University Liquid-Solid Phase Diagrams Solid and liquid phases can be present below the boiling point (e.g., immiscible pair of metals right up to their melting points (As and Bi) 2-component liquid at temperature a1: (1) a1 6 a2 System enters "Liquid+B" pure solid B comes out of solution, remaining ...
› ternary-phase-diagramTernary Phase Diagram - an overview | ScienceDirect Topics According to the Mg–Ag binary phase diagram, the maximum equilibrium solid solubility of Ag in Mg is ∼15 wt% at the eutectic temperature of 472 °C, and it falls to approximately 2 wt% at room temperature, and the intermetallic phase at the Mg-rich side of the phase diagram is Mg 3 Ag (space group P6 3 /mmc, a = 0.488 nm, c = 0.779 nm).

Liquid vapor phase diagram
PDF Phase Diagrams - University of Cincinnati Liquid/Vapor Equilibria Ideal Solid/Liquid Equilibria Ideal Eutectic Non-Ideal Congruent melting solid solution Phase diagram is split into two phase diagrams with a special composition that acts as a pure component Same crystallographic structure in solid solution phase Different crystallographic structures in solid solution phases 12 L => a L ... PDF Phase Diagrams and the Triple Point Phase Diagrams and the Triple Point Consider an isolated (adiabatic) container of water at 100° C. This container has only water, in vapor and liquid form—no air, or any other substance. In this container: • The vapor is in equilibrium with the liquid; that is, if one watches the container, the amounts of vapor and liquid do not change. chem.libretexts.org › Courses › Oregon_Institute_of8.1: Heating Curves and Phase Changes - Chemistry LibreTexts A distinct boundary between the more dense liquid and the less dense gas is clearly observed. As we increase the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water (Figure \(\PageIndex{3}\)), and a two-phase equilibrium of liquid and gaseous phases remains.
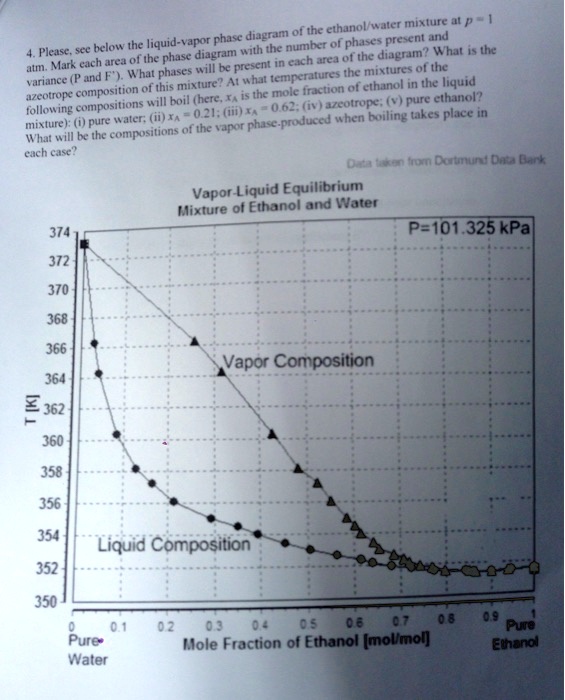
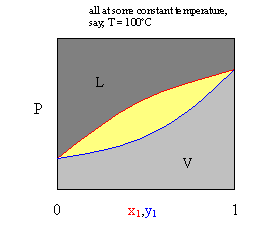
Liquid vapor phase diagram. Binary phase diagrams - PetroWiki Binary phase diagrams. Fig. 1 is a pressure-composition (p-x-y) phase diagram that shows typical vapor/liquid phase behavior for a binary system at a fixed temperature below the critical temperature of both components. At pressures below the vapor pressure of Component 2, p v2, any mixture of the two components forms a single vapor phase.At pressures between p v1 and p v2, two phases can ... Liquid/Vapor phase diagram for ethanol/water solution ... Liquid/Vapor phase diagram for ethanol/water solution. Post by rubelstrudel » Tue Sep 10, 2019 11:04 am I am using the liquid/vapor phase diagram for ethanol/water solution all the time. It gives me all the information I need about my still by just measuring the temp in the top of the column while running. I found this nice chart by googling: 10.4 Phase Diagrams | Chemistry - Lumen Learning The curve BC in Figure 2 is the plot of vapor pressure versus temperature as described in the previous module of this chapter. This "liquid-vapor" curve separates the liquid and gaseous regions of the phase diagram and provides the boiling point for water at any pressure. For example, at 1 atm, the boiling point is 100 °C. › template-for-triangularTemplate for triangular diagrams in MS Excel : Phasediagram Vapor-liquid equilibrium, solid-liquid equilibrium, liquid-liquid equilibrium and others. The data for the three components can be given in mol percent or mass percent. The template was used for making the triangular diagram to the right and for diagrams on several other pages such as the phase diagrams for the mixed solvent systems .
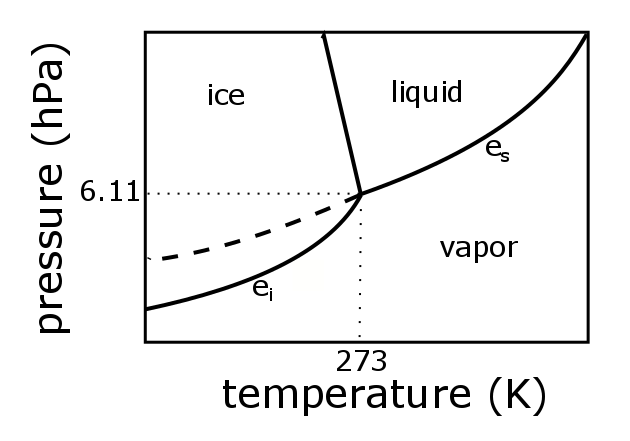
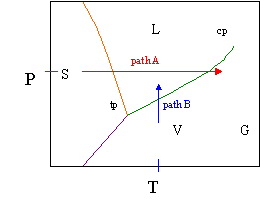
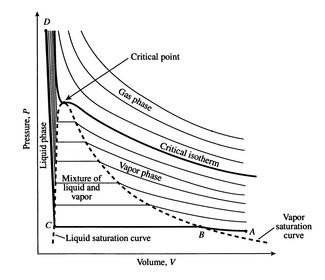
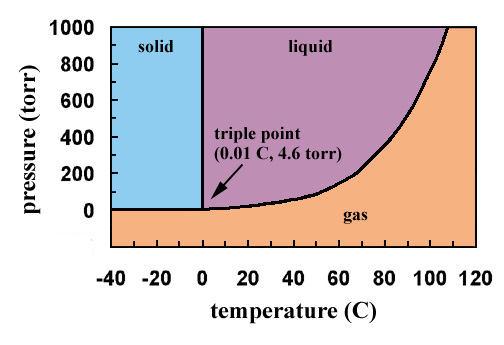
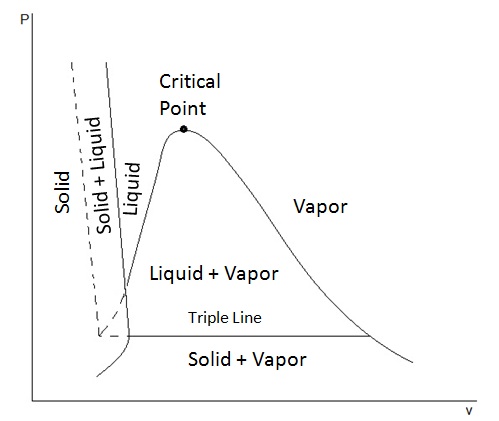
› cms › lib4Answers to Phase Diagram Worksheet - Livingston Public Schools The TRIPLE POINT is the condition of temperature and pressure where ALL THREE phases exist in equilibrium (solid, liquid, gas) Remember that pressure can be expressed in many units where: 1 atm = 101.3 k a = 760 mmH Refer to the phase diagram below when answering the questions on the back of this worksheet: PDF Binary Liquid-Vapor Equilib4 - CPP We will call the values for the liquid phase wt% PrOH, l, for the vapor phase wt% PrOH, v. Notice that for every value of T you have two values of wt% (liquid phase and vapor phase). Arrange the data from both parts A and B above into one set, sorted in order of increasing wt% PrOH. › meteo300 › node3.3 Phase Diagram for Water Vapor: Clausius Clapeyron ... The Phase Diagram for Water We can draw the phase diagram for water. There are three equilibrium lines that meet at the triple point, where all three phases exist ( es = 6.1 hPa; T = 273.14 K). Along the line for es, vapor and liquid are in equilibrium, and evaporation balances condensation. PDF Chapter 3 Thermodynamic Properties the vapor phase is a saturated vapor. Liquid water continues to evaporate with additional heat until it becomes all saturated vapor at point (g). Any further heating will cause an increase in both temperature and specific volume and the saturated vapor becomes superheated vapor denoted by point (s) in Figure 3.2-2. For a two-phase liquid-vapor ...
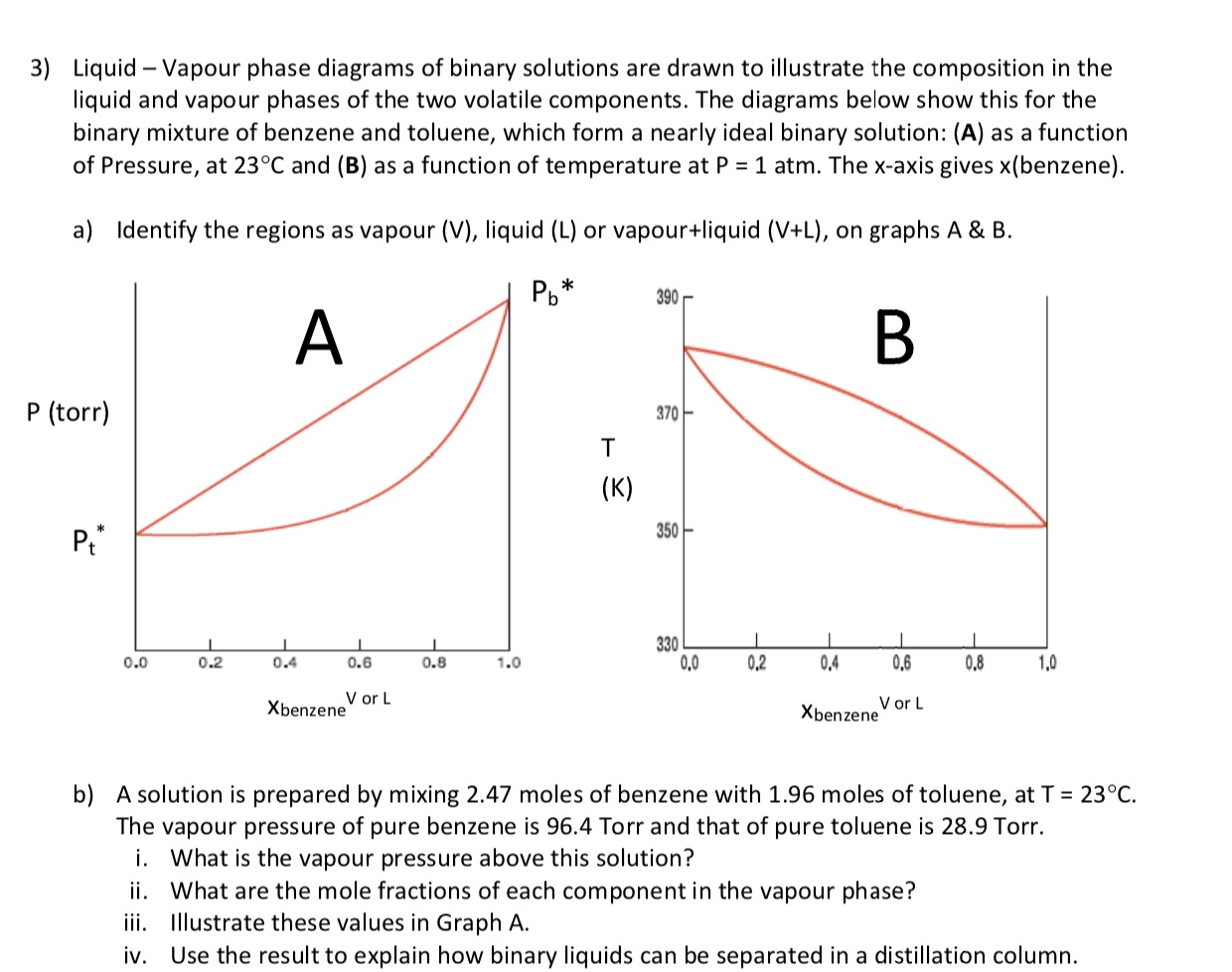
PDF Phase Diagrams State Principle and Quality Evaluating ... Phase Determination (Case 1 Cont'd) • Case 1: Given P and T - Look up saturation table - Compare given P and T against saturation values in the table ¾In temperature table, Recall constant temperature line on P-v diagram If P > P sat(T), compressed liquid. If P = P sat(T), saturated liquid-vapor mixture. If P < P sat(T), superheated vapor. Liquid-Vapor Equilibria in Binary Systems Construct a phase diagram using temperature versus mole percent composition. In the present case, there will be two compositions plotted at each equilibrium ...12 pages PDF Binary Solid-Liquid Phase Diagram Introduction Expt. 5: Binary Phase Diagram CHEM 366 V-1 Binary Solid-Liquid Phase Diagram Introduction The substances that we encounter in the material world are hardly ever pure chemical compounds but rather mixtures of two or more such compounds. The individual substances in such a mixture may behave more or less independent of each other but merely ... Pchem I Lab 4 Binary Liquid-Vapor Phase Diagram.docx - Lab ... Distillation has a close importance to liquid-vapor phase diagrams, and boiling point diagrams. Distillation consists of boiling a liquid and condensing the vapor into a receiver which will lead to the partial or complete separation of a liquid into its components [1].
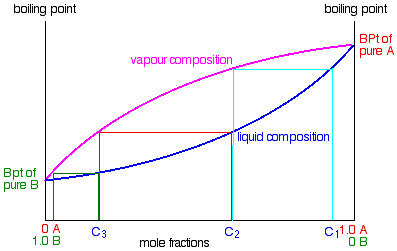
PDF Fundamentals of Vapor-liquid Equilibrium (Vle) 1.2 BINARY VLE PHASE DIAGRAMS There are two types of VLE diagrams that are widely used to represent data for two- component(binary)systems.Thefirstisa"temperatureversusxandy"diagram(Txy).The x term represents the liquid composition, usually in terms of mole fraction. The y term represents the vapor composition.
Lab 5 Binary Liquid-Vapor Phase Diagram.docx - Running ... BINARY LIQUID-VAPOR PHASE DIAGRAM 2 Abstract The goal of the lab is to construct a binary liquid-vapor phase diagram using refractometry and the heterogeneous equilibrium between two phases in a system of two components. Also, the idea of Raoult's Law was applied to the experiment to determine the negative relationships in two diagrams.
chemdictionary.org › phase-diagramPhase Diagram | Explanation, Definition, Summary & Facts Oct 14, 2019 · But in case of water, liquid phase transforms into a solid phase (ice) and solid phase then converts into the vapor phase. Fig. 6 Phase diagram of water is showing that it has a triple point (T) at very low pressure and its critical point temperature is 374 ᵒC, it meant that above this temperature it is almost impossible to convert gas phase ...
PDF Fundamentals of Vapor-liquid Phase Equilibrium (Vle) 1.2 BINARY VLE PHASE DIAGRAMS Two types of vapor-liquid equilibrium diagrams are widely used to represent data for two-component (binary) systems. The first is a "temperature versus x and y" diagram (Txy). The x term represents the liquid composition, usually expressed in terms of mole fraction. The y term represents the vapor composition.
Liquid-Vapor Equilibrium of a Binary System | Chem Lab Place all liquids in the appropriate waste solvent container. Remove and discard the septum. Examine the septum liner, and discard it if damaged. Results and Analysis Prepare a liquid-vapor phase diagram (temperature as a function of the liquid's composition). It is highly recommended that you prepare this diagram as you obtain the data.
study.com › academy › lessonPhase Diagram of Water vs Other Substances: Differences ... Nov 05, 2021 · When the phase diagram for a substance has a solid-liquid boundary line that has a negative slope (leans to the left), the substance: a) can go from solid to liquid, within a small temperature range,
Lab Report 3 liquid vapor phase diagram - CHEM111 ... A liquid-vapor phase diagram plots temperature of mixture versus the mole fraction or composition. A phase diagram of 2 components that will have differing molecular interactions causing mixture's boiling point to rise/drop will show an azeotrope, in which there will be 2 lobes that either concave down/up depending on if there's positive ...
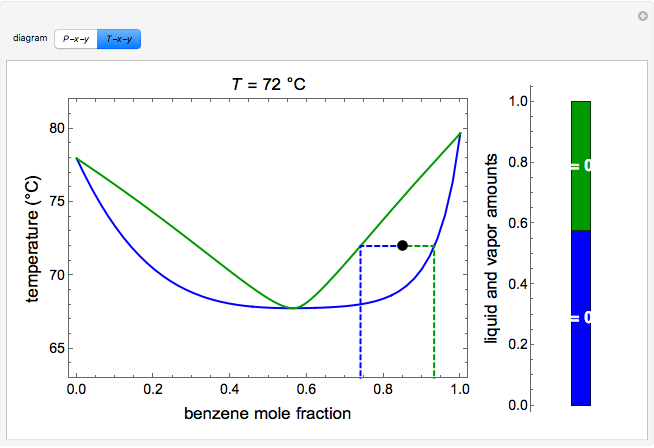
P-x-y and T-x-y Diagrams for Vapor-Liquid Equilibrium (VLE ... The vapor-liquid equilibrium (VLE) behavior of an -hexane/ -octane mixture is demonstrated in and diagrams. The blue line represents the liquid-phase boundary (bubble point) and the green line represents the vapor-phase boundary (dew point). Click and drag the black dot on either diagram and the bar chart shows the amounts of liquid (blue) and ...
10.4 Phase Diagrams - Chemistry: Atoms First 2e | OpenStax A distinct boundary between the more dense liquid and the less dense gas is clearly observed. As we increase the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water (Figure 10.31), and a two-phase equilibrium of liquid and gaseous phases remains. At a temperature of 374 °C ...
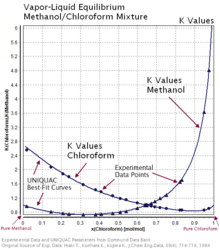
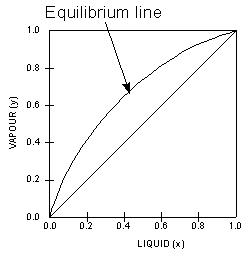
Vapor-liquid equilibrium - Wikipedia Vapor-liquid equilibrium diagrams Vapor-Liquid Equilibrium Diagram For each component in a binary mixture, one could make a vapor-liquid equilibrium diagram. Such a diagram would graph liquid mole fraction on a horizontal axis and vapor mole fraction on a vertical axis.
For vapor to solid/liquid the lines in the phase diagram ... Answer (1 of 2): The term vapor pressure is the pressure of the gas phase over either the liquid or solid phase of a substance. I will explain further using a specific example. CO2 forms a solid (dry ice) when the pressure is high enough and the temperature low enough. This means we can put a pie...
Vapor-Liquid-Liquid Equilibrium (VLLE) - Wolfram ... Because of the partial miscibility, vapor-liquid equilibrium (VLE), liquid-liquid equilibrium (LLE), and vapor-liquid-liquid equilibrium (VLLE) are present on the phase diagram. You can vary the mole fraction of component B and the heat added (location of the black dot).
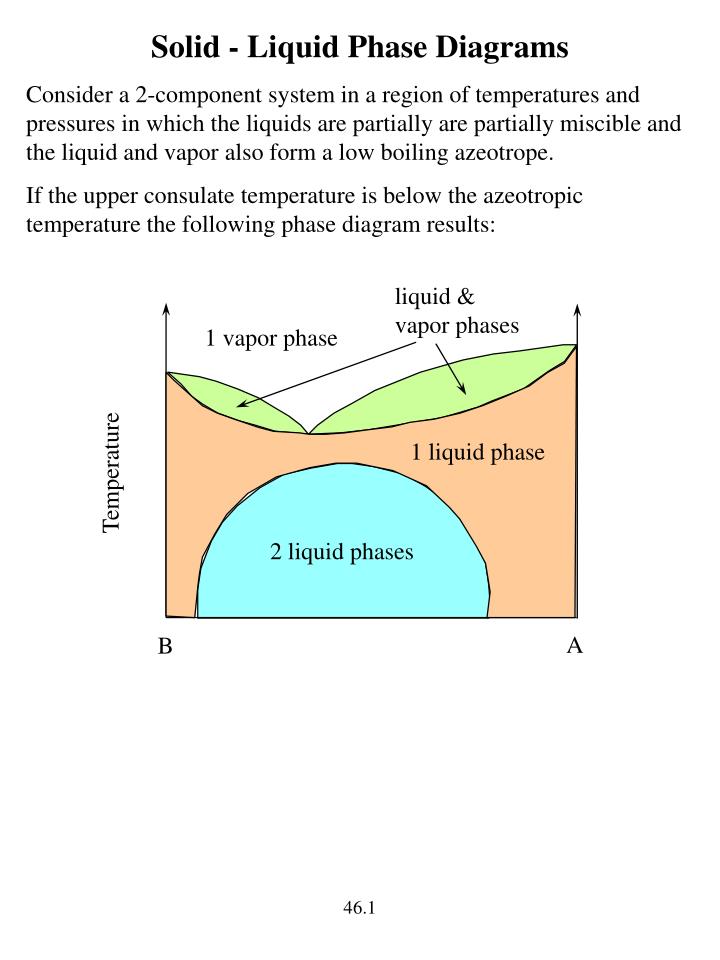
PDF 5.8 Liquid-Liquid phase diagrams 5.8 Liquid-Liquid phase diagrams Key points 1. Phase separation of partially miscible liquids may occur when T is below UCST or above LCST 2. UCST is the highest T at which phase separation occurs. LCST is the lowest T at which phase separation occurs 3. The outcome of a distillation of a low-boiling ...
A Study of Azeotrope and Acetone/Chloroform Liquid-Vapor ... A Study of Azeotrope and Acetone/Chloroform Liquid-Vapor Phase Diagram Abstract: Liquid-vapor phase of acetone/chloroform was studied through distilling a series of mixtures with different mole fraction. When the mixtures were boiling, their vapor was condensed through a water column and collected in a receiving container.
Vapor Liquid Phase Diagrams - Christian Brothers University Vapor Liquid Phase Diagrams Vapor-liquid equilibrium data may be obtained by experiment, by thermodynamic calculation, or in published sources. It is typically presented either in tabular form or as an equilbrium diagram. Diagrams may take several forms: boiling point diagrams Txy, Pxy diagrams ternary diagrams solubility diagrams
Solid-liquid Phase Diagram | USP Technologies Solid-liquid Phase Diagram . Ref: P.A. Giguere. "Complements au Nouveau Traite de C. himie Minerale - No. 4 - Peroxyde d'Hydrogene et Polyoxydes d'Hydrogene" Paris, Masson 1975 (181 p).
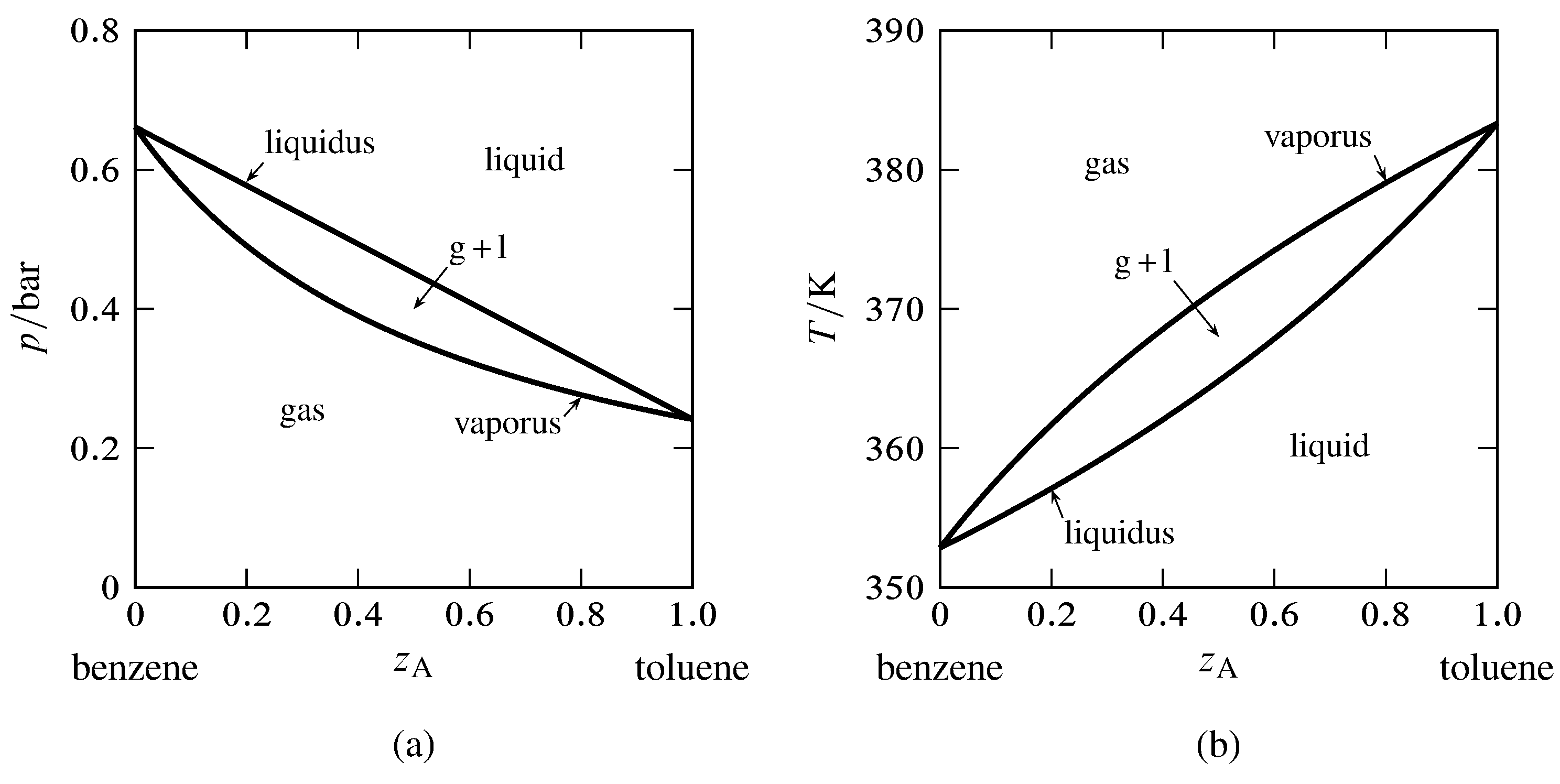
Chapter 8 Phase Diagrams - Central Michigan University Chapter 8 Phase Diagrams. (b) The interpretation of diagrams. Point a represents the vapor pressure of a mixture with liquid composition xA and b represents the composition of the vapor that is in equilibrium with the liquid at that pressure. Note that when two phases are in equilibrium, P = 2, so F' = 1.
Using a Phase Diagram for Vapor Pressure Curves - Swagelok In some analytical systems, a liquid sample must be converted to gas through vaporization before it can be analyzed. Vaporization is essentially a balancing act between temperature, pressure and flow variables - and a phase diagram's vapor pressure curves allow engineers to identify phase changes for distinct materials and chemical compounds.
Determination of a liquid-vapor phase diagram - ACS ... by P Schienbein · 2017 · Cited by 28 — Using RPBE-D3, we approximately map the liquid–vapor phase diagram based on aiGEMC simulations (33−35) and roughly estimate the CP of “RPBE-D3 ...SPECIAL ISSUE: This article is part of the Benj...
chem.libretexts.org › Courses › Oregon_Institute_of8.1: Heating Curves and Phase Changes - Chemistry LibreTexts A distinct boundary between the more dense liquid and the less dense gas is clearly observed. As we increase the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water (Figure \(\PageIndex{3}\)), and a two-phase equilibrium of liquid and gaseous phases remains.
PDF Phase Diagrams and the Triple Point Phase Diagrams and the Triple Point Consider an isolated (adiabatic) container of water at 100° C. This container has only water, in vapor and liquid form—no air, or any other substance. In this container: • The vapor is in equilibrium with the liquid; that is, if one watches the container, the amounts of vapor and liquid do not change.
PDF Phase Diagrams - University of Cincinnati Liquid/Vapor Equilibria Ideal Solid/Liquid Equilibria Ideal Eutectic Non-Ideal Congruent melting solid solution Phase diagram is split into two phase diagrams with a special composition that acts as a pure component Same crystallographic structure in solid solution phase Different crystallographic structures in solid solution phases 12 L => a L ...


















0 Response to "39 liquid vapor phase diagram"
Post a Comment