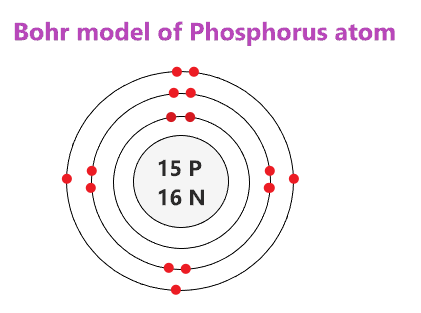
40 bohr diagram of fluorine
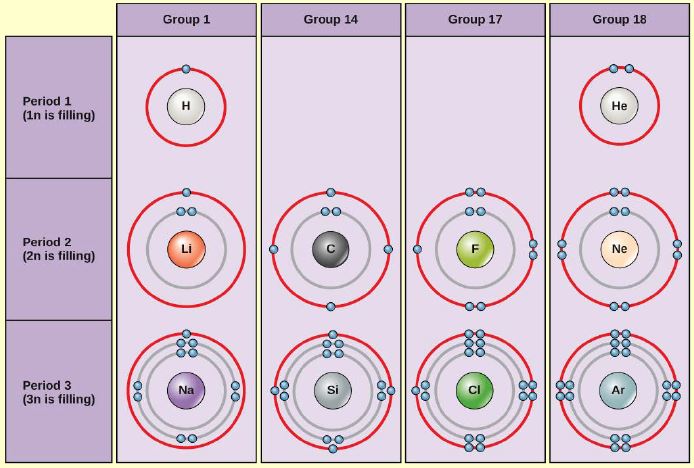
Bohr Rutherford Diagram For The First 20 Elements Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ... Bohr Model of all Elements (Diagrams + Chart Inside) Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of Silicon (Si) 2, 8, 4: 15: Bohr ...
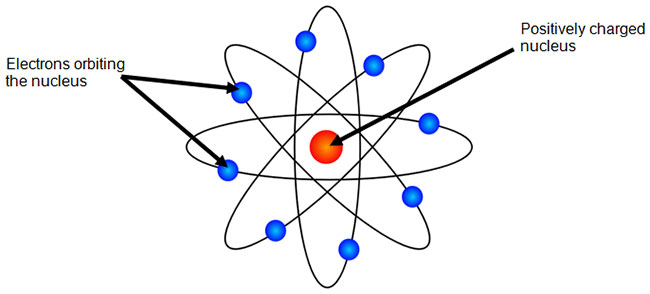
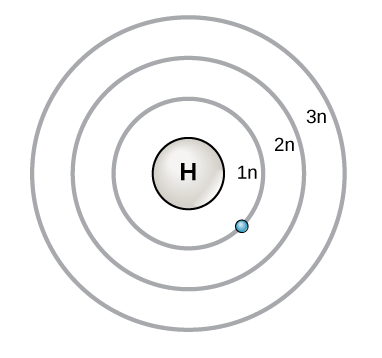
Understanding the Bohr Atomic Model - PrepScholar Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
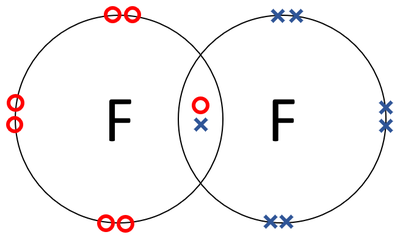
Bohr diagram of fluorine
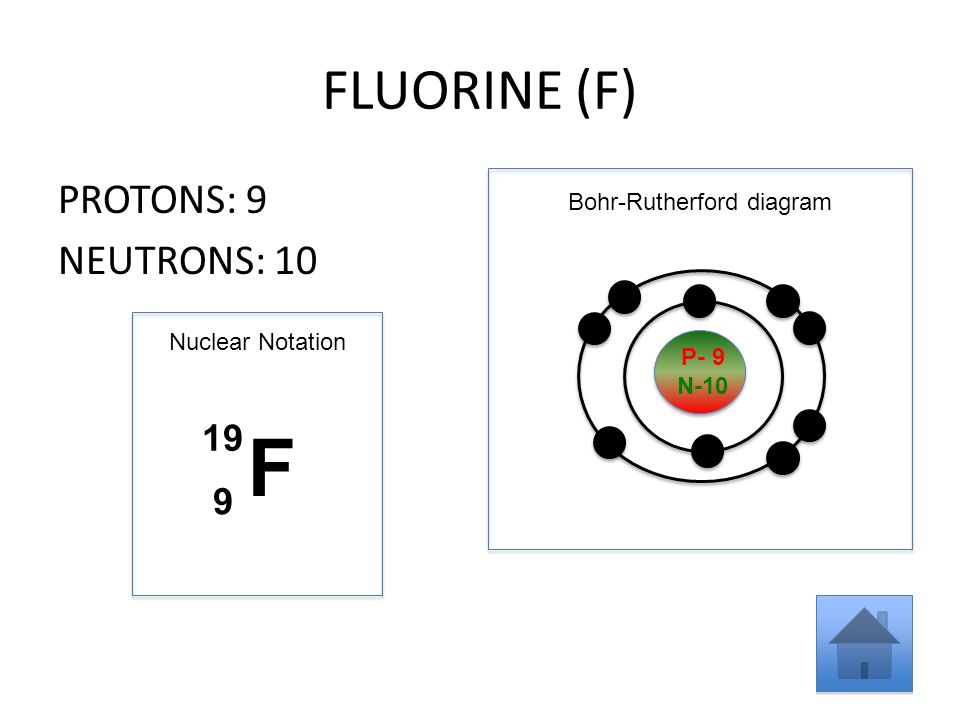
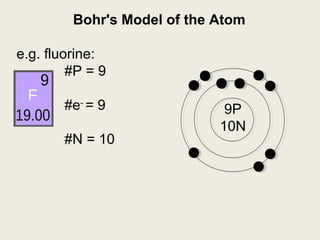
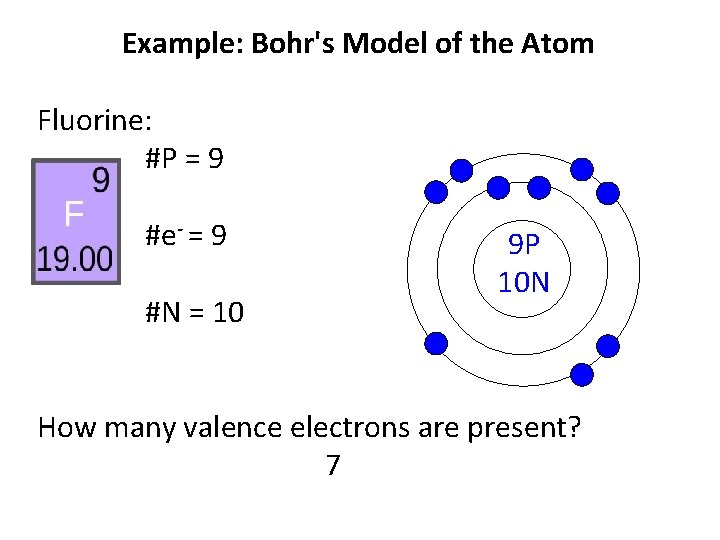
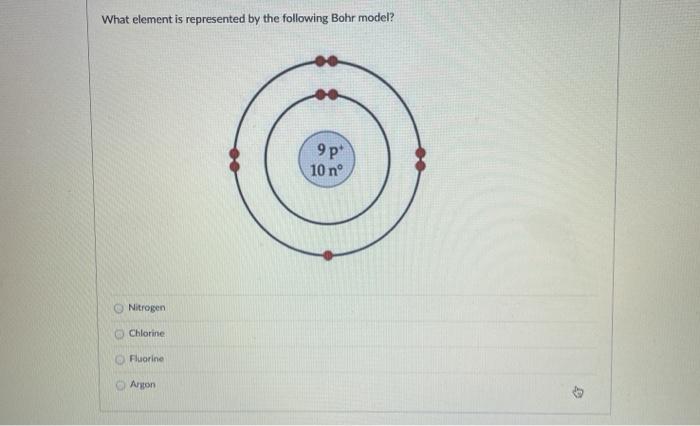
Question: What Is The Bohr Model Of Fluorine ... What is the Bohr configuration of an atom of fluorine? Electron Configuration Standard Notation For example, the electron configuration of lithium is 1s 2 2s 1.The number and letter describe the energy level and orbital, and the number above the orbital shows how many electrons are in that orbital. Bohr diagram for fluorine? - Answers Bohr diagram for fluorine? - Answers Fluorine has an atomic number of 9. protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass number of 19). There will be an... What is the Bohr model of fluorine? - FindAnyAnswer.com According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. Click to see full answer Accordingly, what is the Bohr model for Argon? Here is a bohr model for Argon: We draw dots to represent the electrons.
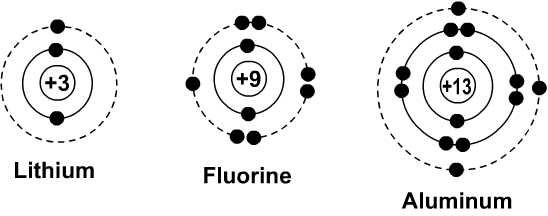
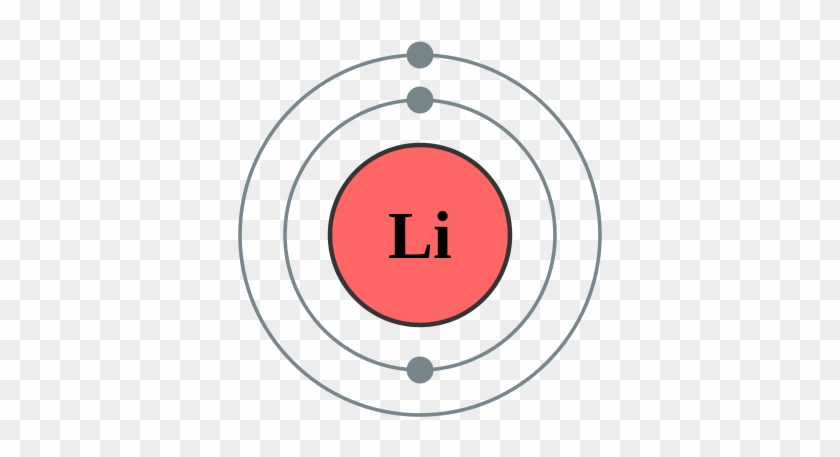
Bohr diagram of fluorine. Potassium Bohr Model - How to draw Bohr diagram for Potassium ... Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Potassium atom with some simple steps. Steps to draw the Bohr Model of Potassium atom. 1. Find the number of protons, electrons, and neutrons in the Potassium 41 fluorine bohr diagram - daughterofarab.blogspot.com Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. Diagram Of Fluorine Atom - Association AVH DIAGRAM OF FLUORINE ATOM album depeche mode blasphemous rumours, titeuf le film, thor le film, rio le film, peter pan le film, lol le film, le film thor, le film de justin bieber, le film 300, le film 2012, ashbahan, manual sugar cane juicer, le filet restaurant montreal, le filet de sole, kyle ashbaugh, jason ashbaugh, dennis ashbaugh, dena ashbaugh, billy ashbaugh, ovation ashbaland, mona ... 8.2 Hybrid Atomic Orbitals – Chemistry To bond six fluorine atoms, the 3s orbital, the three 3p orbitals, and two of the 3d orbitals form six equivalent sp 3 d 2 hybrid orbitals, each directed toward a different corner of an octahedron. Other atoms that exhibit sp 3 d 2 hybridization include the phosphorus atom in PCl 6 − , the iodine atom in the interhalogens IF 6 + , IF 5 , ICl 4 − , IF 4 − and the xenon atom in XeF 4 .
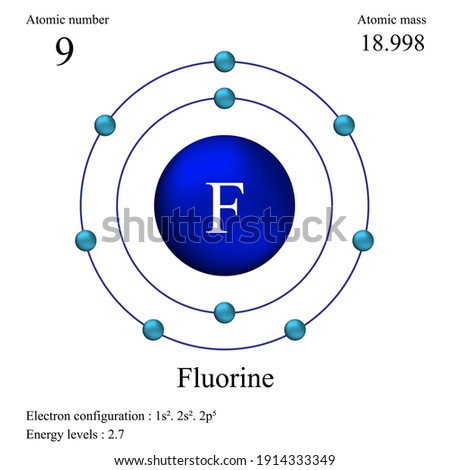
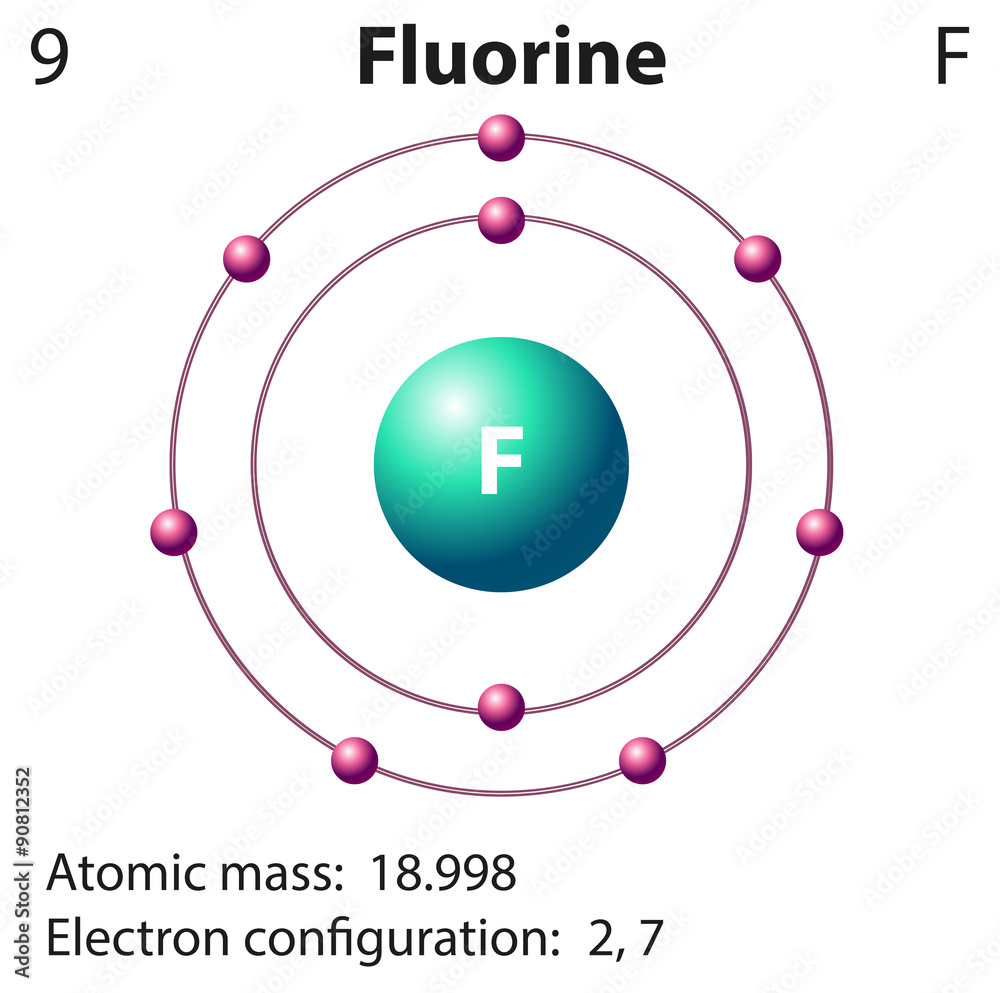
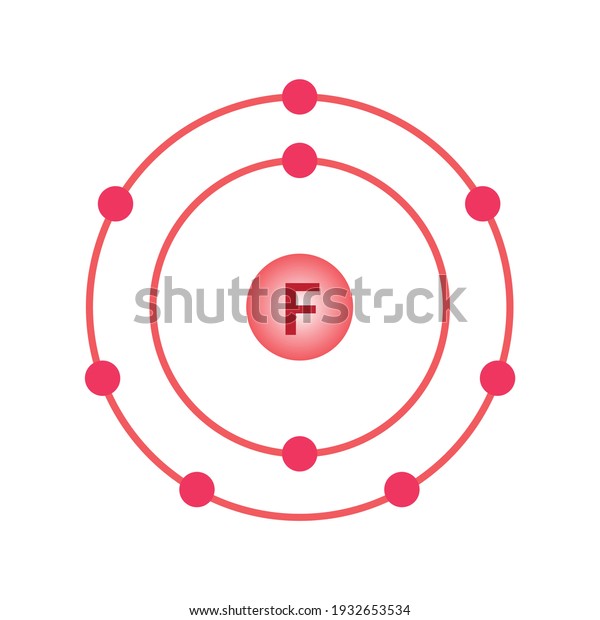
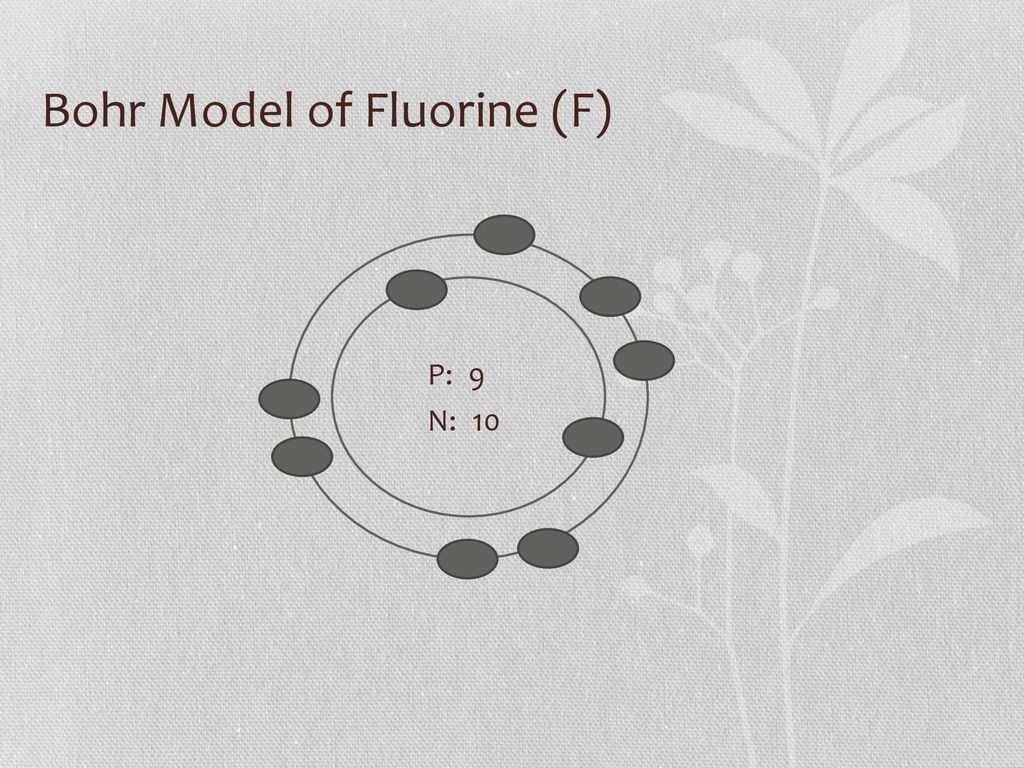
Bohr model of the atom - Chemistry Resource Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits: In the first orbit, there are 2 electrons. In the second orbit, there are 7 electrons. Sulfur Bohr Model - How to draw Bohr diagram for Sulfur (S ... Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Sulfur atom with some simple steps. Steps to draw the Bohr Model of Sulfur atom . 1. Find the number of protons, electrons, and neutrons in the Sulfur atom. Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom … Lewis Dot Structures Worksheet - Mr. Walsh's Class Bohr Diagram. Lewis Dot Structure. Ne. electron config # protons. #electrons. charge. Bohr Diagram. Lewis Dot . Making Ions – Ionic Bonds are made of Ions. A strong understanding of Ions is needed. Notes: Remember that Metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive “cations”. Nonmetals tend to gain electrons, … Fluorine(F) electron configuration and orbital diagram Fluorine (F) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
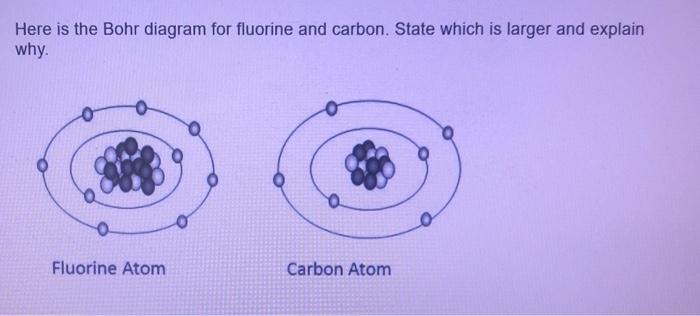
Draw Bohr diagrams of a magnesium atom bonding with ... Draw Bohr diagrams of a magnesium atom bonding with fluorine atoms. Draw atoms then electron transfer and finally the ions that form. What type of bonding occurs? Draw Bohr diagrams of 2 hyrdogen atoms bonding with an oxygen atom by sharing electrons to . Chemistry. Hi! Can you tell me if I chose the right options for my chemistry homework? PDF How to Draw Bohr Diagrams Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li KM 654e-20150109102424 - Columbia Public Schools Bohr Model Drawing Draw a Bohr model of a chlorine atom in the space below. Be sure to place the electrons in the correct orbitals and to fill out the key for the subatomic particles. Protons: Neutrons: Electrons: Chlorine 35.42 Atomic number equals the number of or Atomic mass equals the number of Identify the each of the parts of the box. Oxygen
Bohr Diagram Of Flourine - schematron.org Fluorine has an atomic number of 9. This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass.Figure \ (\PageIndex {2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell.
Term symbol - Wikipedia Term symbols with LS coupling. For light atoms, the spin–orbit interaction (or coupling) is small so that the total orbital angular momentum L and total spin S are good quantum numbers.The interaction between L and S is known as LS coupling, Russell–Saunders coupling (named after Henry Norris Russell and Frederick Albert Saunders, who described this in 1925.
Fluorine Orbital diagram, Electron configuration, and ... The orbital diagram for Fluorine is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Fluorine orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest five electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Fluorine atom is shown below-
Bohr diagram for fluorine? - Answers Bohr diagram for fluorine? - Answers Fluorine has an atomic number of 9. protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass number of 19). There will be an...
Manganese(Mn) electron configuration and orbital diagram Electrons can be arranged correctly through orbits from elements 1 to 18. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram.
how to make a bohr diagram - shapovmusic.com Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. …. Set up the diagram. To set up the diagram, you will need a circle in the middle. …. Add in orbitals and electrons.
Beautiful Bohr Diagram For Argon - Glaucoma Template Bohr Model Diagram Cards Bohr Model Super Teacher Worksheets Homeschool Kindergarten . Figure 2 contrast the Bohr diagrams for lithium fluorine and aluminum atoms. Bohr diagram for argon. Last class we determined that the Bohr Model is a planetary model in which the For example there are 3 shells in the bohr diagram of Argon.
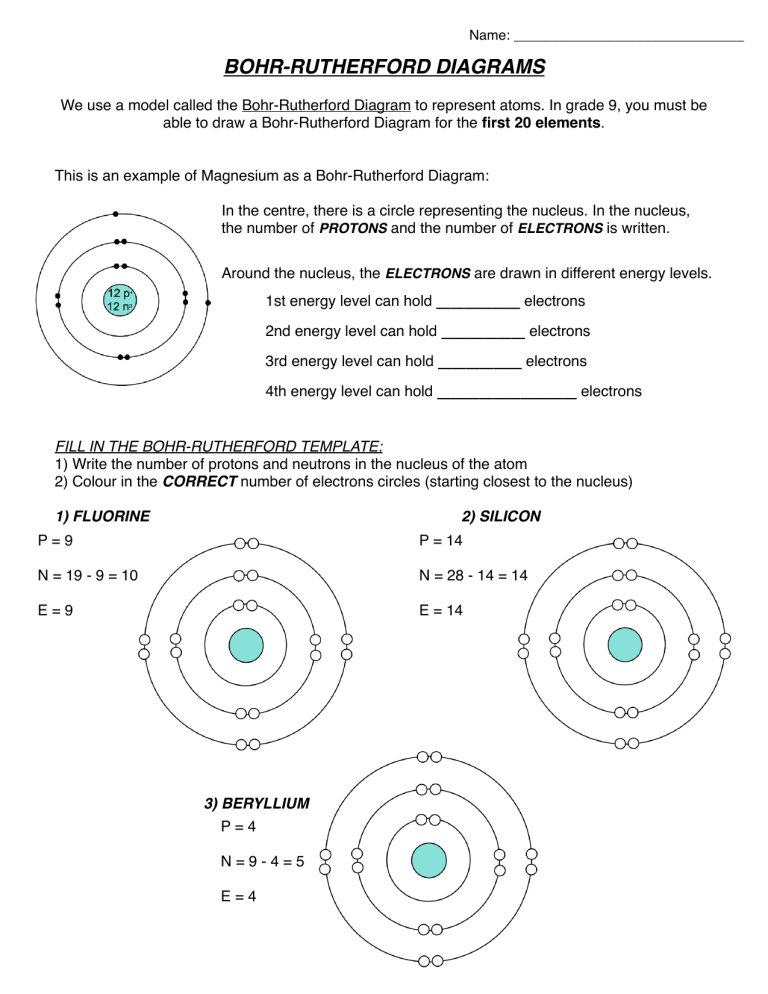
How to Draw the Bohr-Rutherford Diagram of Magnesium Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.Check me out:
Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts Aug 15, 2020 · Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure \(\PageIndex{2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms.
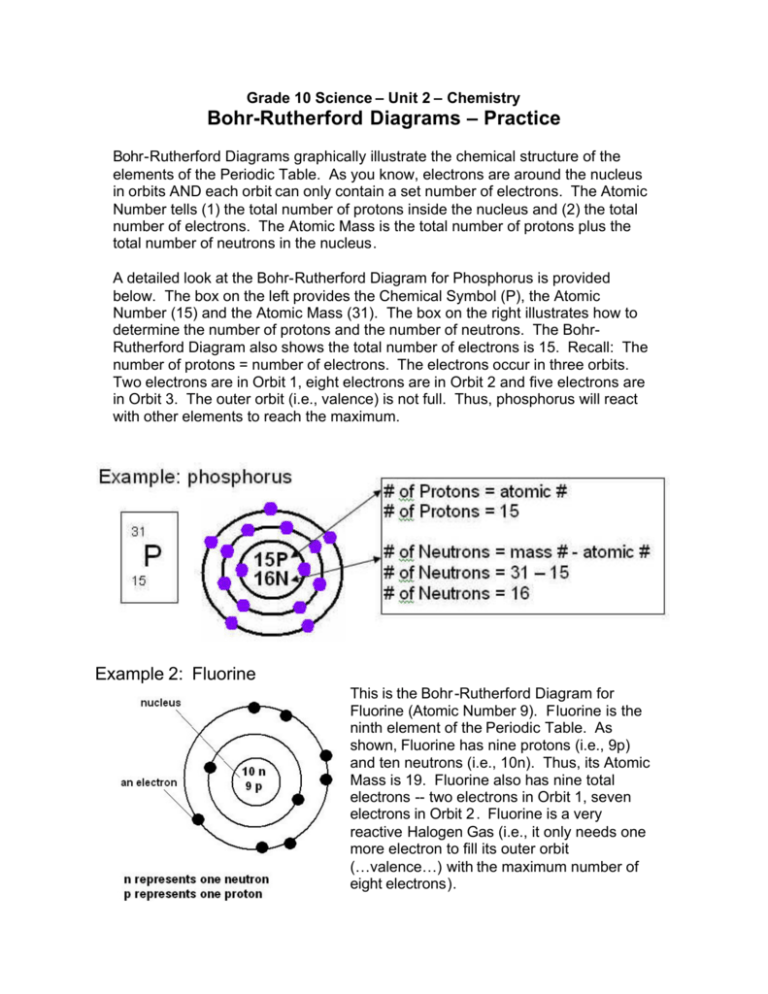
How to Draw the Bohr-Rutherford Diagram of Fluorine - YouTube Fluorine has 2 electrons in its first shell and 7 in its second.Check me out:
Lakhmir Singh Chemistry Class 10 Solutions Periodic ... (e) The modern periodic table was prepared by Bohr . Lakhmir Singh Chemistry Class 10 Solutions Page No:284. Question 42: The atomic masses of three elements X, Y and Z having similar chemical properties are 7, 23 and 39 respectively. (a) Calculate the average atomic mass of elements X and Z.
ATOMIC STRUCTURE - users.stlcc.edu According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons.
Electron Configurations & The Periodic Table By clicking on the NaCl diagram, a model of this crystal ... These illustrations use a simple Bohr notation, with valence electrons designated by colored dots. Note that in the first case both hydrogen atoms achieve a helium-like pair of 1s-electrons by sharing. In the other examples carbon, oxygen and fluorine achieve neon-like valence octets by a similar sharing of electron …
Nitrogen Bohr Model - How to draw Bohr diagram for Nitrogen(N ... The Bohr Model of Nitrogen(N) has a nucleus that contains 7 neutrons and 7 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Nitrogen contains 5 electrons that also called valence electrons.
Diagrams - Public Service Announcement: Sodium Fluoride Lewis Dot Diagram- The sodium transfers one electron to the fluorine so they can both become stable. Bohr-Rutherford Diagram: Sodium is the cation as it has a positive charge after losing one electron, while fluoride is the anion as it has a negative charge after gaining one electron. The two atoms attract, as they have opposite charges.
Bohr Diagram For Fluorine - schematron.org According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 .
Fluorine Bohr Model - How to draw Bohr diagram for ... Bohr's diagram of Fluorine has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell. Hence, the electrons found in the L-shell of the Fluorine atom are its valence electrons because it is the outermost shell that also called the valence shell.
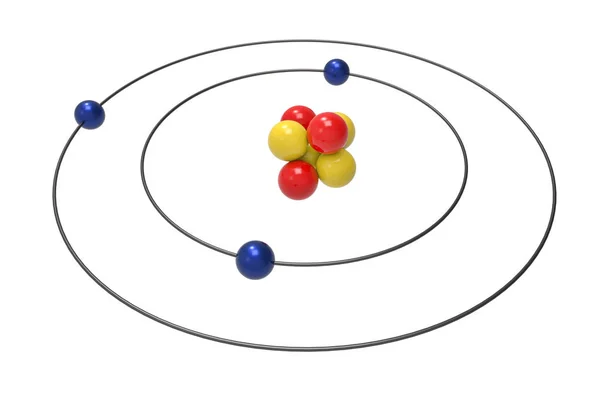
Pictures: bohr model of fluorine | Bohr Model Fluorine ... Photo "Bohr model of Fluorine Atom with proton, neutron and electron. Science and chemical concept 3d illustration" can be used for personal and commercial purposes according to the conditions of the purchased Royalty-free license. The image is available for download in high resolution quality up to 10000x6670.
Draw a Bohr-Rutherford diagram for hydrogen fluoride ... Step 1. 1 of 2. The diagram below shows a fluorine molecule (F. 2 _2 2 . ) with a total of 18 electrons. Result. 2 of 2. The diagram below shows a fluorine molecule (F. 2 _2 2 .
Carbon Bohr Model - How to draw Bohr diagram for Carbon(C ... Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Carbon atom with some simple steps. Steps to draw the Bohr Model of Carbon atom . 1. Find the number of protons, electrons, and neutrons in the Carbon atom. Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the …
What is the Bohr model of fluorine? - FindAnyAnswer.com According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. Click to see full answer Accordingly, what is the Bohr model for Argon? Here is a bohr model for Argon: We draw dots to represent the electrons.
Bohr diagram for fluorine? - Answers Bohr diagram for fluorine? - Answers Fluorine has an atomic number of 9. protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass number of 19). There will be an...
Question: What Is The Bohr Model Of Fluorine ... What is the Bohr configuration of an atom of fluorine? Electron Configuration Standard Notation For example, the electron configuration of lithium is 1s 2 2s 1.The number and letter describe the energy level and orbital, and the number above the orbital shows how many electrons are in that orbital.















0 Response to "40 bohr diagram of fluorine"
Post a Comment