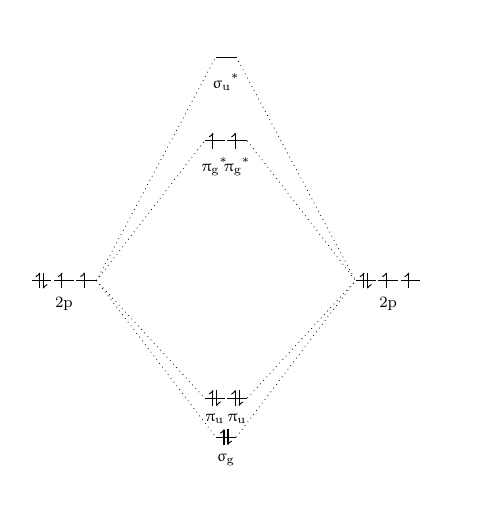
40 molecular orbital diagram for o2 2
software - How to calculate molecular orbitals of ... This question about octaoxygen:. Why is octaoxygen diamagnetic?, was asked in chemistry, but I really don't have an answer in terms of molecular orbitals. I think it could be a mixing of the O $_2$ 's $\pi^*$ orbitals that have been bent inwards the monoclinic structure due to the high pressure of the $\varepsilon$-phase (about 10 GPa), thus greatly reducing sample volume, but I can't find a ... How To Write Electronic Configuration In Molecular Orbital ... In molecular orbital theory, O2+ contains 15 electrons & it contains only one electron. orbitals, we find 17 electrons as well as 3 electrons. Stable molecules consist of fewer electrons with orbitals in antibonding orientation.
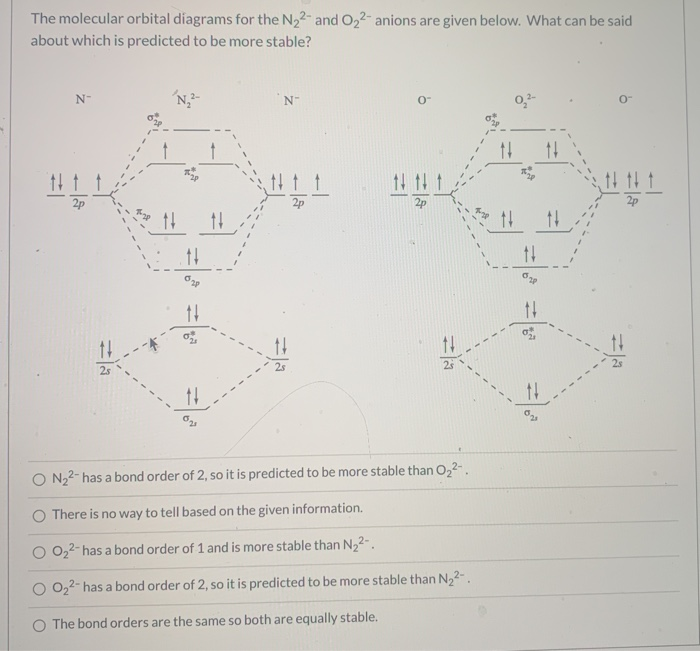
Use the molecular orbital diagram shown to determine which ... The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule.
Molecular orbital diagram for o2 2
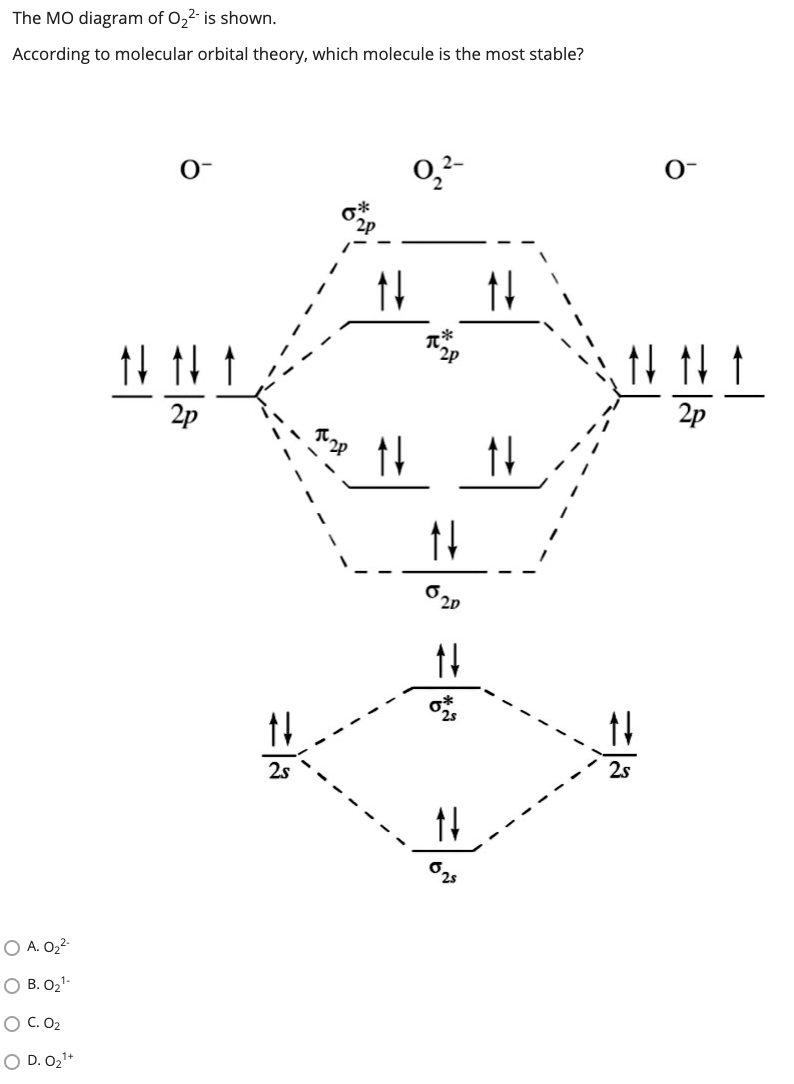
What Is The Bond Order Of O2 Minus Minus The energy diagram of O2molecule is: The electrons in π∗2Px and π∗2Py remain unpaired. So, there are two unpaired electrons in O2. Hence, it is paramagnetic in nature with two unpaired electrons. Is O2 1 paramagnetic or diamagnetic? Oxygen is paramagnetic mainly because it consists of two unpaired electrons in its last molecular orbital. What Is The Bond Order Of O2 Molecular oxygen, O 2, is photolyzed by light of 241 nm and has a bond energy of 498 kJ/mol. Hydrogen peroxide, HOOH, has a very weak O-O bond and is photolyzed by light of 845 nm. Its bond energy is only about 142 kJ/mol. Why do we see such large difference in the strength of oxygen - oxygen bonds in these molecules . 8 - Drawing Molecular Orbital Diagrams — Flux Science Well, s-p mixing doesn't occur with diatomic oxygen, creating a molecular orbital diagram like the first in this article. This is because, as more electrons are added to a system, the higher the energy becomes, due to their electrostatic repulsion. If the energy of the 2s and 2p orbitals are too far apart, mixing won't occur.
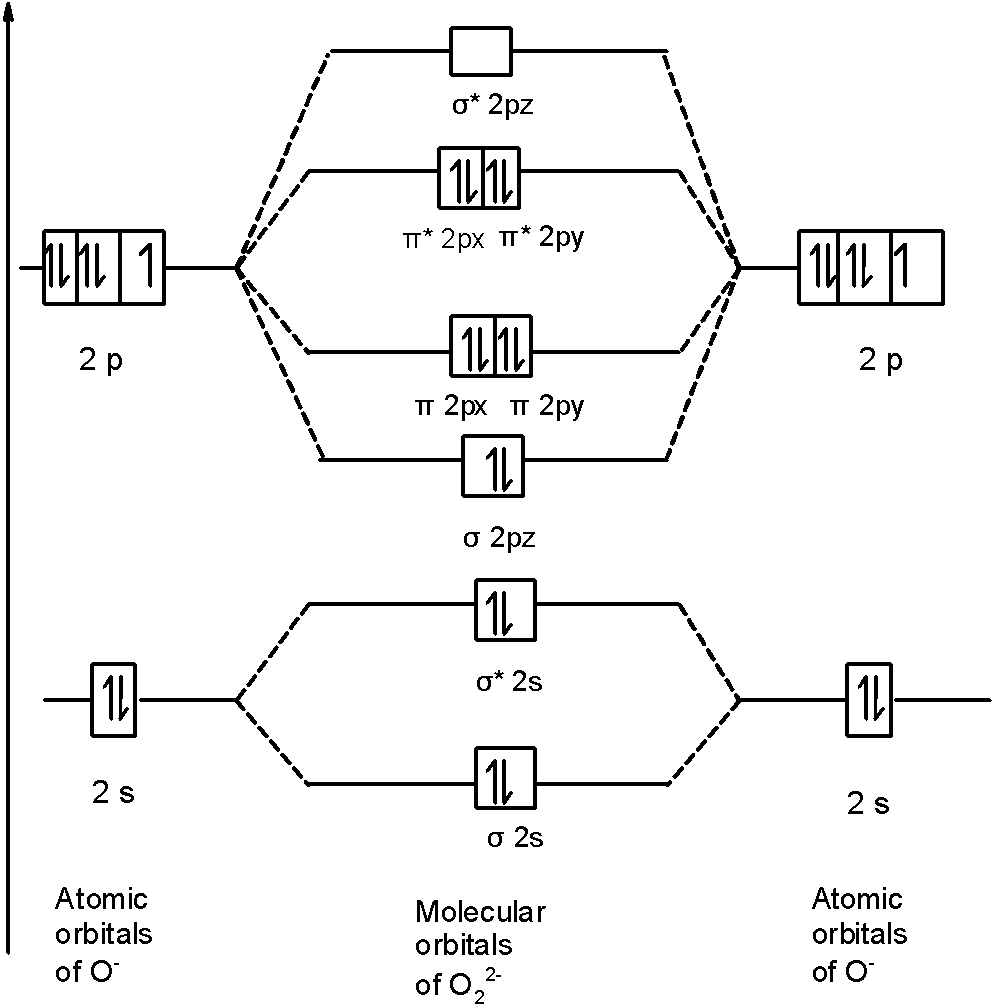
Molecular orbital diagram for o2 2. 40 molecular orbital diagram for he2+ - Diagram For You Construct the molecular orbital diagram for he2 Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each. Therefore, the number of bonding electrons are 2 and the number of ... (Get Answer) - Draw molecular orbital diagrams for O2 ... Which has the highest bond order? Which would. Draw molecular orbital diagrams for O2-, O22-, and O2. Which has the highest bond order? Which would be paramagnetic, and which would be diamagnetic? Can you draw good dot structures that correspond to each of these ions or molecules? Mar 22 2022 05:53 AM. Solution.pdf. how to draw molecular orbital diagram of o2 - Add My Voice ... The molecular orbitals from lowest energy to highest energy are one sigma 2 s orbital one sigma 2 s star orbital two pi 2 p orbitals one sigma 2 p orbital. Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are Molecular Orbital Theory MCQs With FREE PDF b) A molecular orbital is singly occupied. c) An example is oxygen molecule. d) Repelled by the magnetic field. Answer: Repelled by the magnetic field. 8. Combination of two atomic orbitals results in the formation of two molecular orbitals namely. a) one bonding and one non-bonding orbital. b) two bonding orbitals. c) two non-bonding orbitals
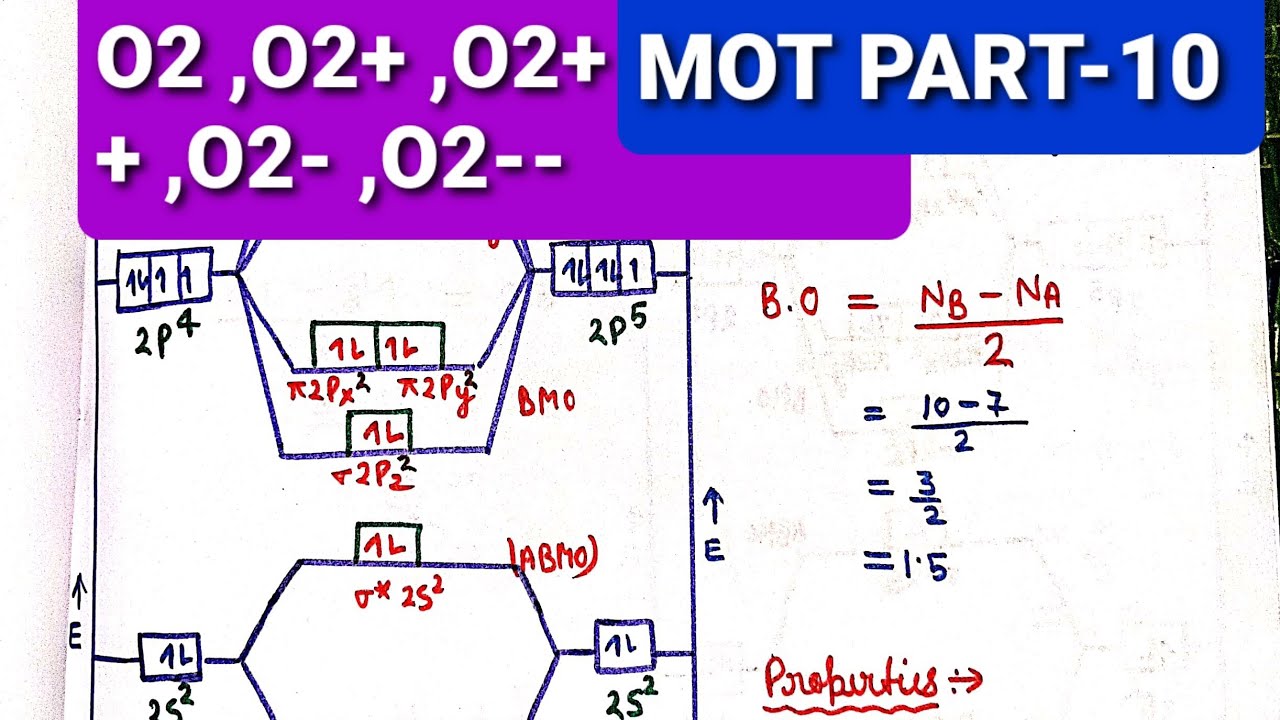
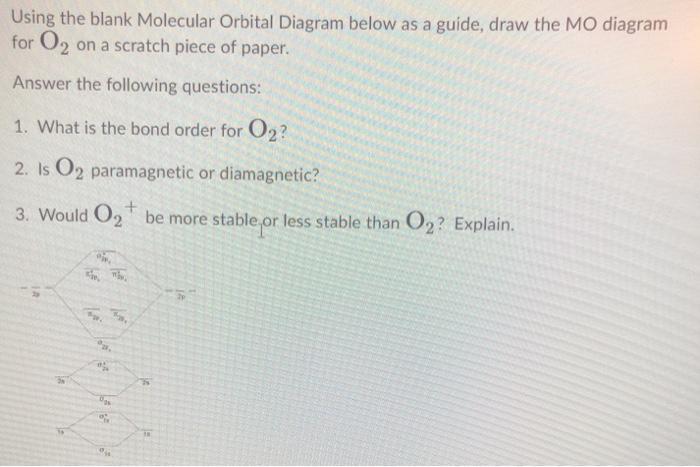
F2 Molecular Orbital Diagram - 17 images - what is the ... [F2 Molecular Orbital Diagram] - 17 images - programable molecular orbital states of c60 from, no2 molecular orbital diagram untpikapps, what is the energy level diagram of n2 and f2 chemistry, mo bonding in f2 and o2 chemistry libretexts, Latest Info on SO2 Molecular Geometry - Education Is Around The molecular geometry of sulphur dioxide is a curved shape. Sulphur to the Oxygen ratio in Sulfur dioxide is 1:2. Sulphur dioxide molecule has two double bonds between the Sulfur atom and also Oxygen atoms. There are five only sets of electrons in the molecule of sulphur dioxide. Molar mass of SO2 = 64.066 g/mol. 40 n2 molecular orbital diagram - leizbasi.blogspot.com Draw molecular orbital diagram of O2 or N2 with magnetic ... Solution. , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O. N2+ Mo Diagram A molecular orbital diagram, or MO ... Draw the molecular orbital energy diagram for O2 and ... Transcribed image text : 3 marks 9. The molecular orbital diagram for the O2+ ion is shown below, place the valence electrons, determine whether it is diamagnetic or paramagnetic, and calculate the bond order (show your work). 0 2p T 20 02 02 02...
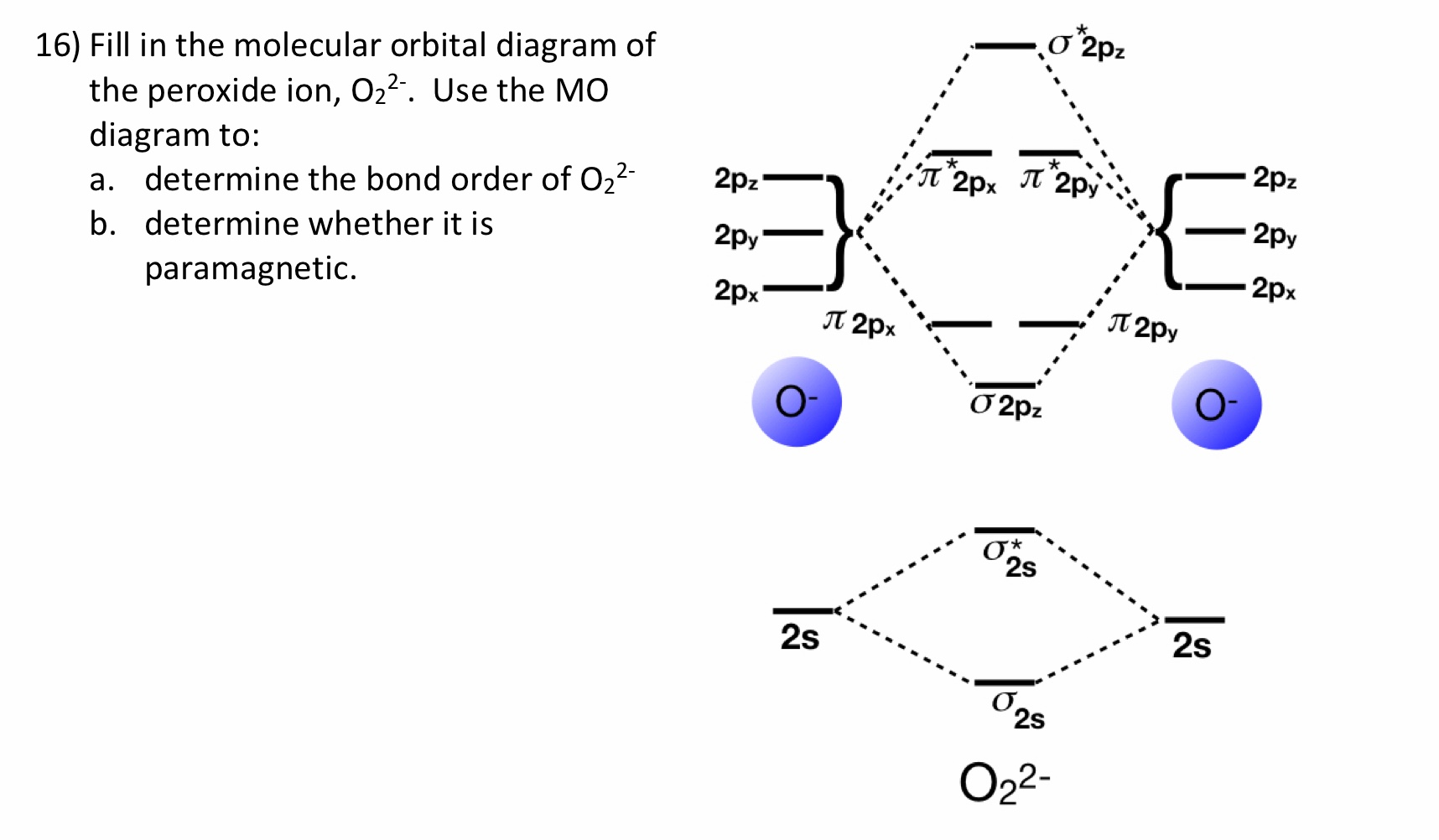
The total number of electrons in all bonding molecular ... The total number of electrons in all bonding molecular orbitals of O2-2 is ..... . (Round off to the nearest integer) What, Is, The, Molecular, Orbital, Diagram, For, Oxygen ... Read Or Download Is The Molecular Orbital Diagram For FREE For Oxygen at DIGIVALEY.COM What Is The Molecular Orbital Diagram Of O2 ... What is the bond order of O2 -? 2 O2 has two unpaired electrons in its π* orbitals, and a bond order of 2. How do you draw a molecular orbital diagram for O2? 0:003:38Molecular Orbital (MO) Diagram of O2 - YouTubeYouTube. How many electrons are in bonding orbitals in O2? 1: Molecular Orbital Energy-Level Diagrams for O2. What is bond order of b2? - All Famous Faqs What is the bond order of O2 and N2? Calculate the bond order of: N 2, O 2,O 2 +,and O 2 -. Bond order is defined as the number of covalent bonds in a covalent molecule. It is equal to one half of the difference between the number of electrons in the bonding and antibonding molecular orbitals. Hence, the bond order of oxygen molecule is 2.
Give the molecular orbital diagram of O2 molecule and ... The stability and magnetic properties of a molecule can be well explained using the molecular orbital theory developed by F Hung and R.S.Mulliken.Calculate the bond order and predict the magnetic property of `O_2` molecule. In O_2 molecule, the correct order of molecular orbitals is 630388947 100+ 2.5 k+ 02:39
39 molecular orbital diagram for o2 2- - Diagram For You Molecular Orbital Theory - Purdue University The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. Molecular Orbitals of the Second Energy Level.
43 o2 2- mo diagram - Modern Wiring Diagram The molecular orbital diagram of an Oxygen molecule is as - N2+ Mo Diagram 2- = Molecular orbital for N2, N2+, O2, H2 and He2 by Thomas Wells - December 5, Brian Verfuerth 0. Ozone Lewis diagrams and by avatar Claire Bridget .
What are the quantum numbers of oxygen ... Why do we need different quantum numbers for molecular orbitals? A quantum description of molecular orbitals require different quantum numbers, because the Hamiltonian and its symmetries are quite different. This describes the electron shell, or energy level, of an electron. The value of n ranges from 1 to the shell containing the outermost ...
What Is The Ground State Electron Configuration And The ... Bond order is defined as the number of covalent bonds in a covalent molecule.It is equal to one half of the difference between the number of electrons in the bonding & antibonding molecular orbitals. Hence, the bond order of oxygen molecule is 2. Thus, the bond order of O 2+ is 2.5.
What is the molecular orbital configuration of C2? 2022 ... Because According to molecular orbital theory O2+ has 15 electrons &it has one electron in antibonding orbital. In the case of O2- 17 electrons are present &3 electrons are present in antibonding orbitals. Why Be2 do not exist? The electronic configuration of Beryllium is 1s2 2s2.
how to draw molecular orbital diagram of n2 - We Had A Big ... The molecular orbital diagram representing this order of energy levels is shown in fig. σ 1 s 2 σ 1 s 2 σ 2 s 2 σ 2 s 2 Π 2 p x 2 Π 2 p y 2 σ 2 p z 2. Same thing would go for a. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule.
Chemical Bonding and Molecular Structure Class 11 ... In the molecular orbital diagram for O 2 + ion, the highest occupied orbital is (a) σ MO orbital (b) π MO orbital (c) π* MO orbital (d) σ* MO orbital. Answer. C. Question. The theory capable of explaining paramagnetic behaviour of oxygen is (a) resonance theory (b) V.S.E.P.R. theory (c) molecular orbital theory (d) valence bond energy ...
Bonding in Metal Carbonyls: Explaination, Type, Property ... Molecular Orbital Diagram of CO. To understand the bonding in metal carbonyls, we need to first learn the Molecular Orbital \(\left( {{\rm{MO}}} \right)\) diagram of carbon monoxide. There are ten electrons in the carbon monoxide ligand. ... The lower energy atomic orbitals of oxygen contribute more to the bonding molecular orbital.
38 molecular orbital diagram for c2 - Diagram Online Source (Use the molecular orbital diagram) B:, C2, N2, O2 A. 0 В. 1 C. 2 D. 3 E. 4 Energy A 30. Use the molecular orbital diagram to figure out the electronic configuration for N2. DOC Molecular Orbitals of Diatomic Molecules - La Salle University This diagram should be to scale. If necessary, you can omit the two lowest molecular orbitals from the ...
8 - Drawing Molecular Orbital Diagrams — Flux Science Well, s-p mixing doesn't occur with diatomic oxygen, creating a molecular orbital diagram like the first in this article. This is because, as more electrons are added to a system, the higher the energy becomes, due to their electrostatic repulsion. If the energy of the 2s and 2p orbitals are too far apart, mixing won't occur.
What Is The Bond Order Of O2 Molecular oxygen, O 2, is photolyzed by light of 241 nm and has a bond energy of 498 kJ/mol. Hydrogen peroxide, HOOH, has a very weak O-O bond and is photolyzed by light of 845 nm. Its bond energy is only about 142 kJ/mol. Why do we see such large difference in the strength of oxygen - oxygen bonds in these molecules .
What Is The Bond Order Of O2 Minus Minus The energy diagram of O2molecule is: The electrons in π∗2Px and π∗2Py remain unpaired. So, there are two unpaired electrons in O2. Hence, it is paramagnetic in nature with two unpaired electrons. Is O2 1 paramagnetic or diamagnetic? Oxygen is paramagnetic mainly because it consists of two unpaired electrons in its last molecular orbital.















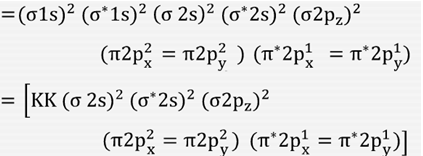









0 Response to "40 molecular orbital diagram for o2 2"
Post a Comment