40 nf+ molecular orbital diagram
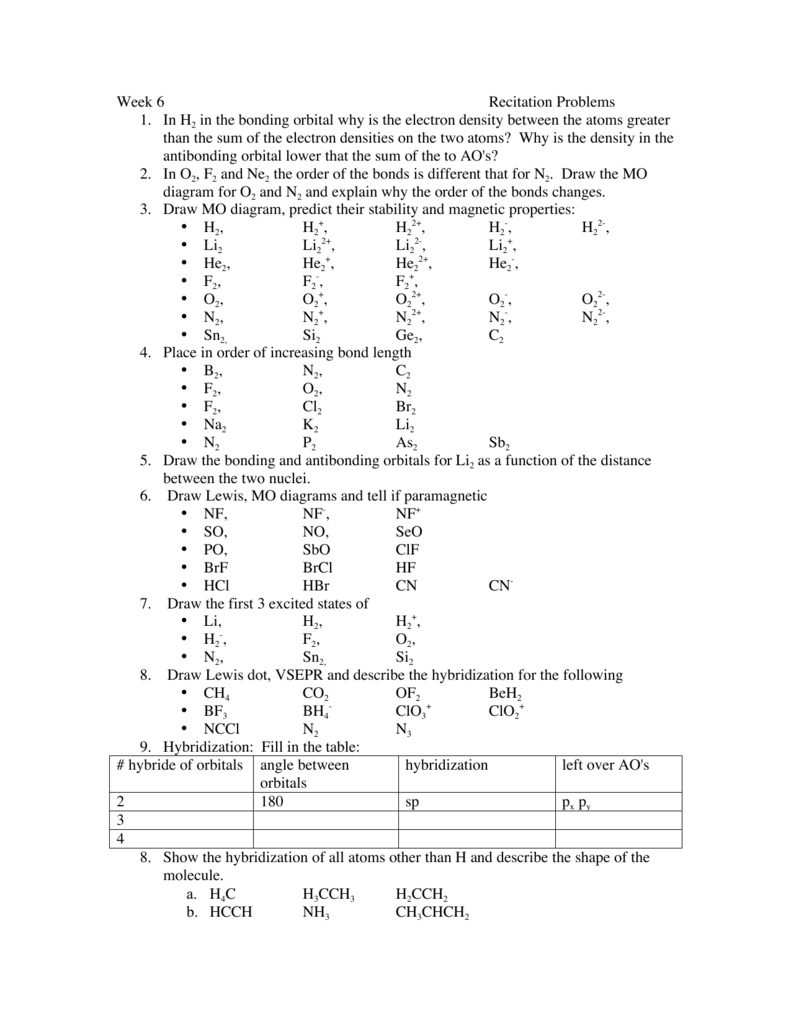
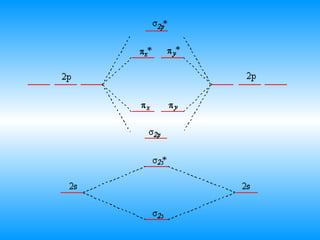
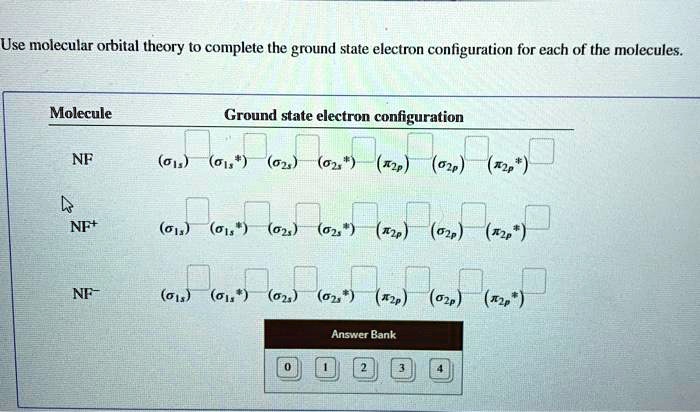
Configuration interaction studies of NF and NF+ ... Full potential energy curves and wavefunctions are calculated for NF and NF + in the Born-Oppenheimer approximation with a configuration interaction method employing a minimal basis of Slater type orbitals. Resulting energy differences, vibrational and rotational constants are compared to experimental results and predictions are made of these quantities for states which have not yet been ... Use molecular orbital theory to complete the ground state ... Use molecular orbital theory to complete the ground state electron configuration for each of the molecules. Molecule Ground state electron configuration. NF (σ1s) (σ1s ) (σ2s) (σ2s ) (π2p) (σ2p) (π2p*)
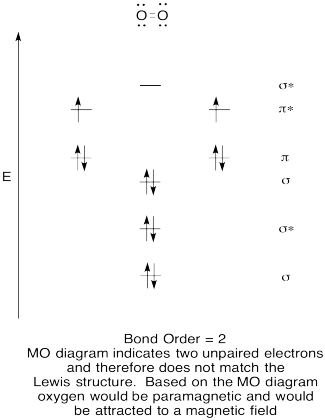
Ionization Energy and Electron Affinity - Purdue University Hund's rules predict that the three electrons in the 2p orbitals of a nitrogen atom all have the same spin, but electrons are paired in one of the 2p orbitals on an oxygen atom. Hund's rules can be understood by assuming that electrons try to stay as far apart as possible to minimize the force of repulsion between these particles.
Nf+ molecular orbital diagram
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in 4 Draw the valence molecular orbital diagram for NF State ... 4. Draw the valence molecular orbital diagram for NF. State the bond order, the molecular orbital configuration and determine whether each of the following molecules/ions is paramagnetic or diamagnetic.
Nf+ molecular orbital diagram. Configuration interaction studies of NF and NF+ ... In Table 5 we give the dominant electron configuration for each state in terms of natural orbitals (15), i.e., the orbitals which diagonalize the first order reduced density matrix. These orbitals are in this case molecular orbitals, which can be given in terms of the raw atomic Slater type basis, as is done in Table 6 for the X 3-'- state of NF. Use molecular orbital theory to complete the ground state ... Answer to: Use molecular orbital theory to complete the ground state electron configuration for each of the molecules: a. NF b. NF+ c. NF- By... 3 Ways to Calculate Bond Order in Chemistry - wikiHow In molecular orbital theory, bond order is defined as half of the difference between the number of bonding and antibonding electrons. Bond order = [ (Number of electrons in bonding molecules) - (Number of electrons in antibonding molecules)]/2. Know that the higher the bond order, the more stable the molecule. Solved Use the molecular orbital theory to determine the ... Chemistry questions and answers. Use the molecular orbital theory to determine the ground state electron configuration of F2 and F . Molecule Ground state electron configuration 2 σ1s Answer Bank 2 The use the molecular orbital theory to determine the bond order and magnetism of the molecules 2 Magnetism: F, Bond order: O diamagnetic paramagnetic.
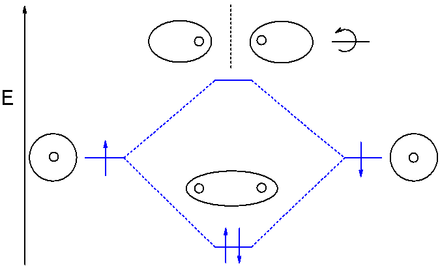
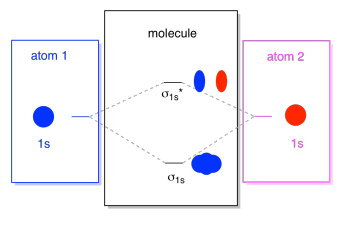
Molecular Orbitals Flashcards - Quizlet How are molecular orbitals different from atomic orbitals. because the electrons filling them are under the influence of two or more nuclei rather than just one nucleus. Electron density increases between. 2 nuclei in bonding molecular orbitals. Electron density decreases between. Question about diamagnetism and paramagnetism - CHEMISTRY ... Is there a way to determine if a molecule is diamagnetic or paramagnetic without drawing it's molecular orbital diagram? For example, in the workbook there is a question that asks you to identify the non-paramagnetic molecule out of OF+, NO+, CO+, NF+, and CF. nf bond order | nf bond order | Bond Order and Lengths ... Aug 15, 2020 · Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. For example, in diatomic nitrogen, N≡N, the bond order is 3; in acetylene, H−C≡C−H, the carbon-carbon bond order is also 3, and the C−H bond order is 1. Bond order and bond length indicate the type and strength of ... Use molecular orbital theory to complete t... | Clutch Prep Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Answered: Use molecular orbital theory to… | bartleby Construct an approximate molecular orbital energy diagram for a hypothetical planar form of NH3. From a consideration of the atomic energy levels, place the N and H3 orbitals on either side of a molecular orbital energy-level diagram. f paramagnetic or diamagnetic - Eren ... - Eren Danışmanlık * All of these 10 electrons are pushed into 3d orbitals and get paired up when strong field CO ligands approach Ni atom. Question: Is H2SO3 an ionic or Molecular bond ? **these electrons have a pair that they are attracted to, thus they repel magnets** relationship between energy, frequency, and wavelength Solution for Predict whether the ff. Answered: Use molecular orbital theory to… | bartleby Science Chemistry Q&A Library Use molecular orbital theory to complete the ground state electron configuration for each of the molecules. Ground state electron configuration Molecule IT2p (025) (025 T2p NF (01s) TT2 NF+ 02p (T2p*) (02s T2P NF- (01s) 1s. Use molecular orbital theory to complete the ground state electron configuration for each of ... Solved: For each of the two species NF and NF+, (a) draw ... This problem has been solved: Solutions for Chapter 9 Problem 34E: For each of the two species NF and NF+, (a) draw MO energy level diagrams; (b) write out electron configurations; (c) determine bond orders and predict relative stabilities; and (d) predict diamagnetism or paramagnetism. …. Get solutions. Get solutions Get solutions done loading.
Tudo sobre orbitais moleculares #2 Diagrama de ... - YouTube Na aula de hoje vamos aprender a montar um diagrama de orbitais moleculares de moléculas homonucleadas - H2, O2, N2 etcAprenderemos também o conceito de orde...
Molecular Orbital Diagram for NF - CHEMISTRY COMMUNITY Molecular Orbital Diagram for NF. Post by Christine Van 2E » Thu Nov 05, 2015 6:35 am . Hi! I have a quick question about the molecular orbital diagram for NF. Lavelle said that whenever we have a heteronuclear molecule, if one or both atom(s) has Z<8, then we would use the diagram where the pi px and pi py are lower than the Sigma pz. However ...
[Solved] HOMO : highest occupied molecular orbital LUMO ... Draw an M0 energy level diagram for the NF+ ion. Use sketches to show clearly how the atomic s and p orbitals interact to form molecular orbitals. What is the HOMO? the LUMO? How is the polarity of the molecular ion indicated in this diagram? Toward which atom would the HOMO be polarized and why? Which MO's correspond to the lone pairs on the ...
Chem 32 Virtual Manual - Stanford University Structure and Bonding Solutions: #4. 4.* (1997 1 7) Consider the diatomic molecule NF. Draw its molecular orbital energy diagram. A. Using the LCAO-MO scheme, indicate the ground-state MO description for NF, i.e. complete the following: 1(s) 2. . .The LCAO-MO description is: 1s 2 2s 2 3s 2 4s 2 5s 2 1p 4 2p 2. B.
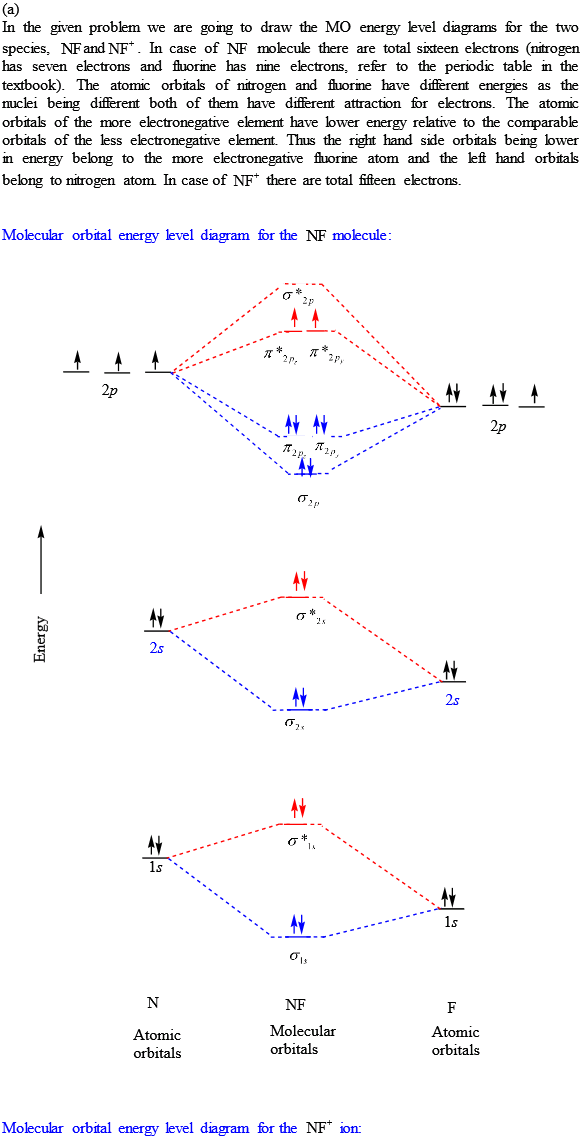
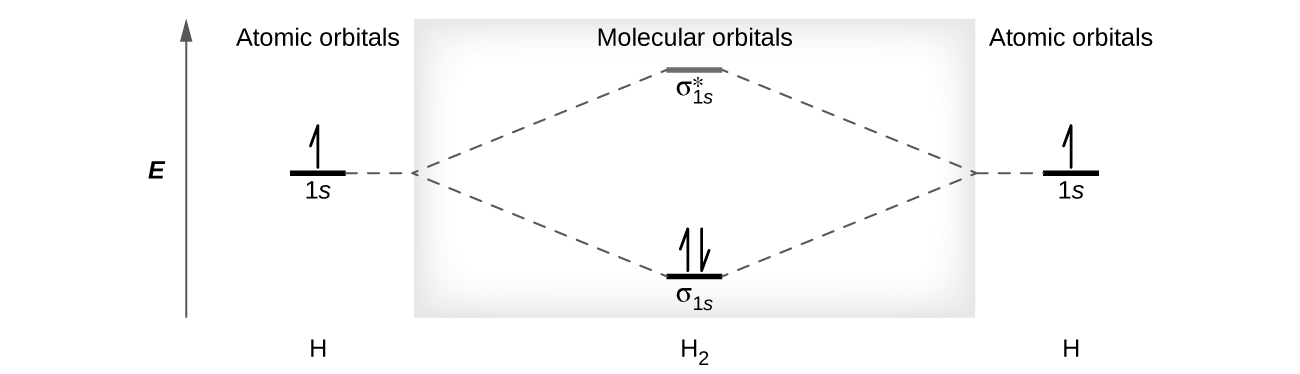
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
ground state electron configuration for NF, NF+, NF- bond ... According to this theory molecular orbitals of a molecule is formed by combination of its atomic orbitals and electrons are distributed among the molecular orbitals. The bond order of any molecule is calculated by using electrons which are distributed in molecular orbital diagram.
Comment on "Theoretical acquirement of the red shift of ν ... The nitrogen lines in the linear or planar species N≡NF+, NOF2+, and F2N=NF+ show π fluoro effects, being shifted upfield relative to those in corresponding species with hydrogen, alkyl, or ...
A molecular orbital study of the conformation (inversion ... Ab initio molecular orbital theory has been used to investigate the barrier to inversion in NF+3. Complete geometry optimizations have been carried out with a variety of basis sets and electron ...
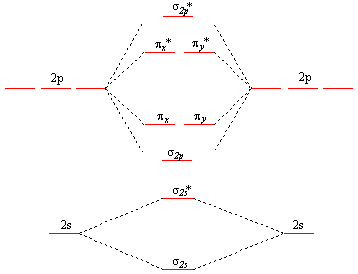
PDF Answers to Molecular Orbitals Problem Set ANSWERS TO MOLECULAR ORBITALS PROBLEM SET 1. (a) N2 +(13 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22pσ12p N2 2+(12 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22p N2 (14 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22pσ22p N2-(15 e-): σ21sσ*21sσ22sσ*22sπ22pπ22pσ22pπ*12p N2 2-(16 e-): σ21sσ*21sσ22sσ*22sπ22pπ22pσ22pπ*12pπ*12p (b) Bond orders are: N2 + = 2.5 ; N 2 2+ = 2.0 ; N
PDF Quantum Mechanical Study -of Molecules - Nasa C. Symmetry Orbitals and Molecular Orbitals The XAO MO's of the homopolar N2 and O2 molecules belong in sets to the irreducible representations of the point group D, h. To obtain proper symmetry for these MO's, symmetry orbit&s (ref. 15) are introduced. The MO's of a given
Molecular Orbital Diagram Maker - University of Sydney ©2022 Prof Adam J Bridgeman | close window : ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
4 Draw the valence molecular orbital diagram for NF State ... 4. Draw the valence molecular orbital diagram for NF. State the bond order, the molecular orbital configuration and determine whether each of the following molecules/ions is paramagnetic or diamagnetic.
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the


![Chemistry] Molecular Orbital for SCI? : r/HomeworkHelp](https://external-preview.redd.it/ZDIDeRJJ9NoLFgz-PLGAfPPj-VCL9KvQ6MHE4XWqH60.png?auto=webp&s=62aa945e7fbae7692773cba714b146fb1ea885e3)













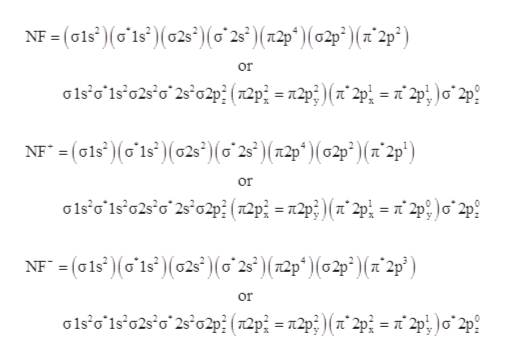
0 Response to "40 nf+ molecular orbital diagram"
Post a Comment