40 orbital diagram for germanium
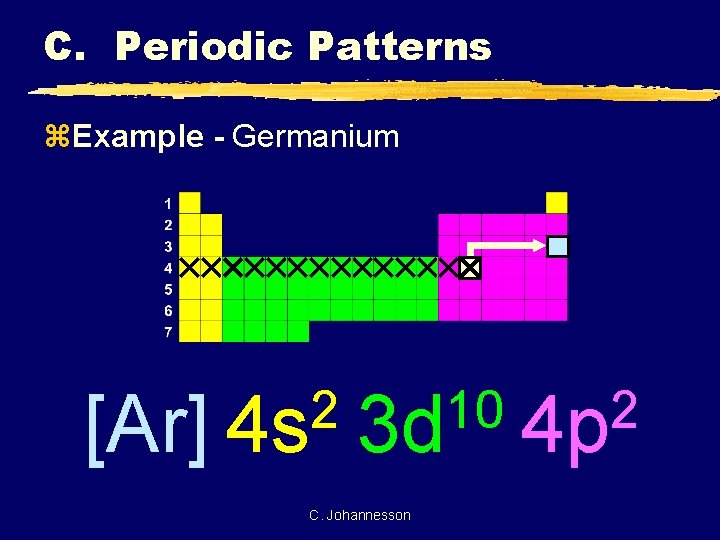
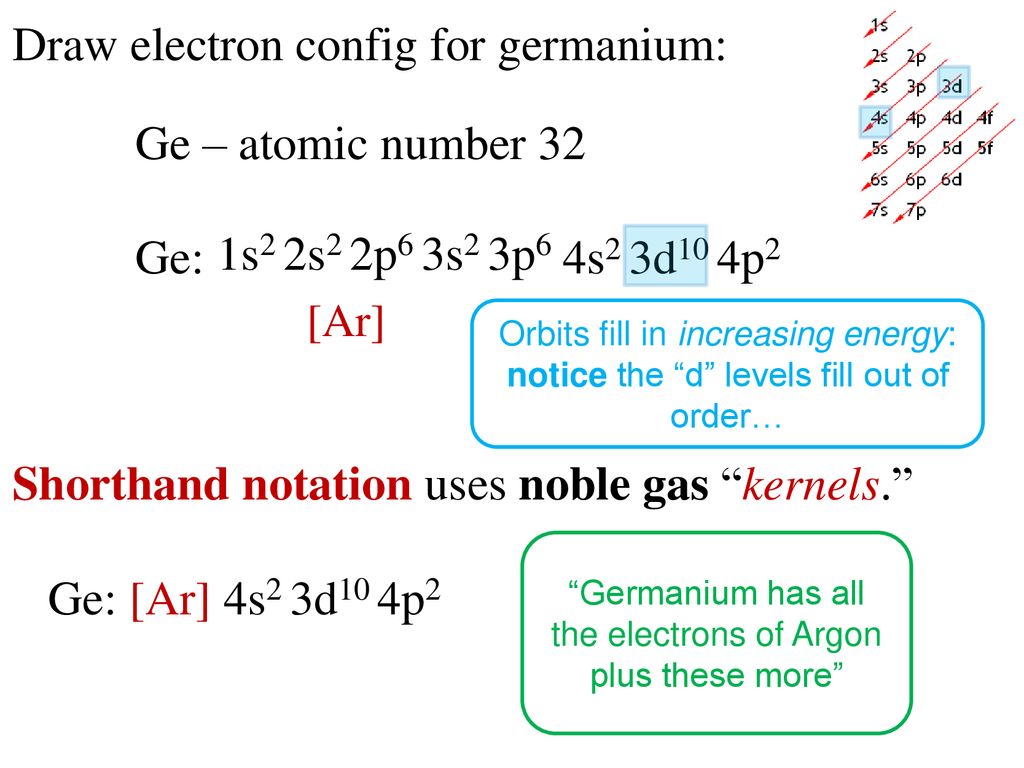
Answered: What is the orbital diagram for the… | bartleby What is the orbital diagram for the atom Geranium Ge 32. Expert Solution. Want to see the full answer? Check out a sample Q&A here. See Solution. Want to see the full answer? Check out a sample Q&A here. See Solution. Want to see this answer and more? Experts are waiting 24/7 to provide step-by-step solutions in as fast as 30 minutes!* What is the ground state electron configuration of the ... Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So, "Ge": 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^(2) An alternative way of writing the electron configuration for Ge is by using the noble gas shorthand notation.
[Solved] Write the orbital diagram for the ground state of ... Write the orbital diagram for the ground state of the germanium atom. Write the orbital diagram for the ground state of the germanium atom. Give all orbitals. Write the orbital diagram for the ground state of cobalt. The electron Write the orbital diagram for the ground state of cobalt. The electron configuration is [Ar]3d74s2.

Orbital diagram for germanium
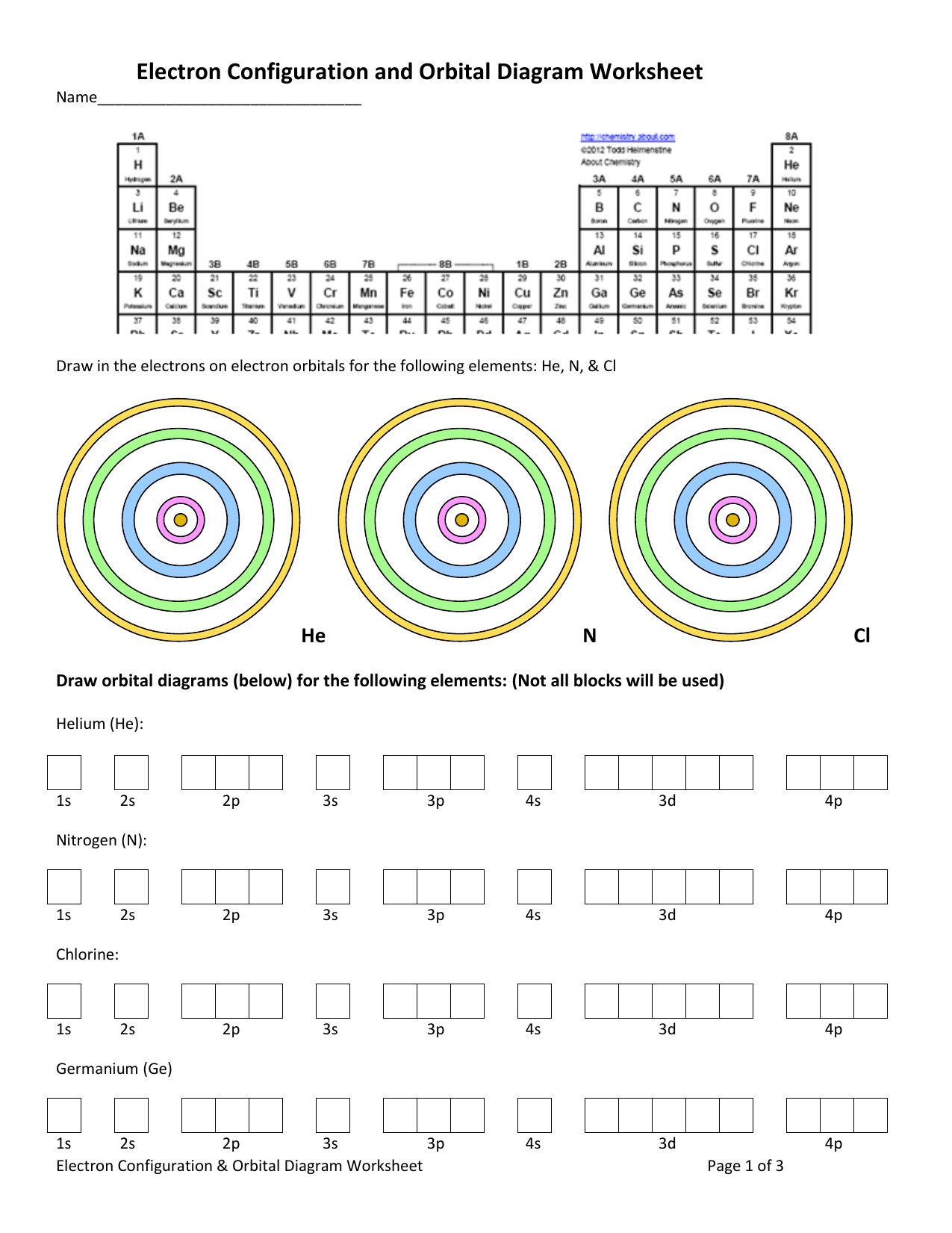
Germanium (Ge) - ChemicalAid Germanium (Ge) has an atomic mass of 32. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? How many electrons are in each shell including 3p orbitals 19.1.2021 · The atom’s electrons exist in atomic orbitals, and we can identify the configuration of each of them using a set of precise guidelines and measures. The Bohr model, named after Neils Bohr, is a one-dimensional model that illustrates the electron’s composition and distribution in the atoms. While you need more information to determine the configuration, … How many …
Orbital diagram for germanium. Metalloid - Wikipedia Germanium analogues of all of the major types of silicates have been prepared. The metallic character of germanium is also suggested by the formation of various oxoacid salts. A phosphate [(HPO 4) 2 Ge·H 2 O] and highly stable trifluoroacetate Ge(OCOCF 3) 4 have been described, as have Ge 2 (SO 4) 2, Ge(ClO 4) 4 and GeH 2 (C 2 O 4) 3. How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. PDF Orbital Diagrams and Electron Configuration Orbital Diagrams and Electron Configuration . Symbol Total number of Orbital Diagram ... Al Ge Cl Mg (metalloid) c) K Li U H (non-metal) d) Ca Ni B I (alkaline earth metal) 9. Use these elements to answer the questions below S, Cl, Al, Na a) Increasing ionization energy b) decreasing electronegativity ... PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
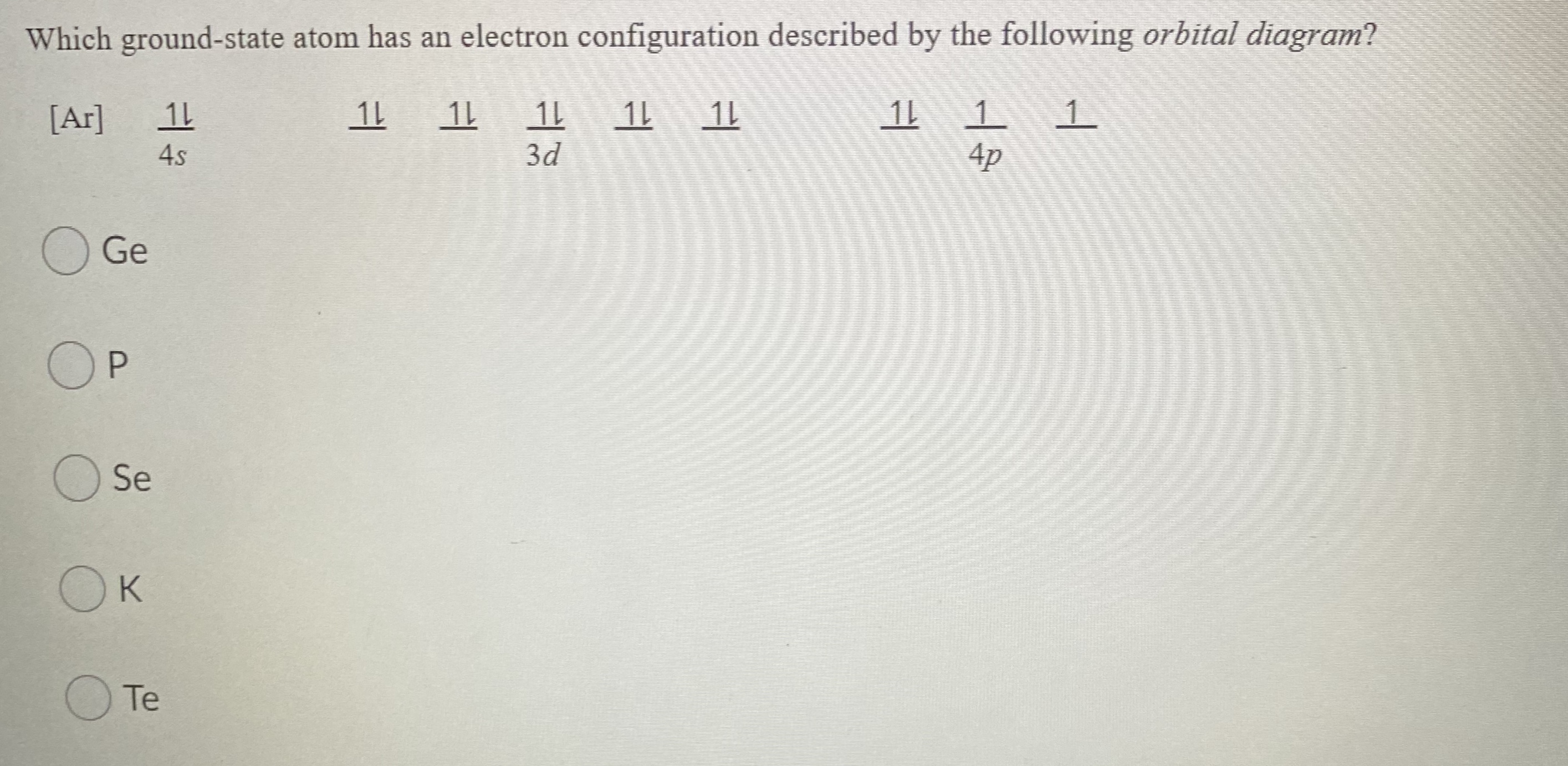
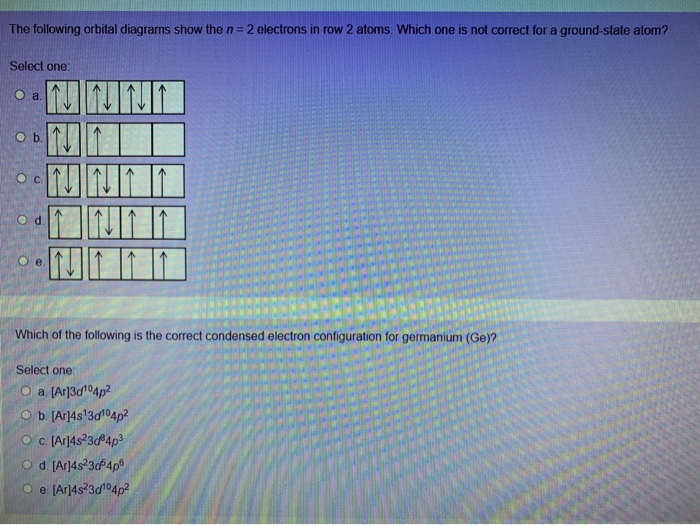
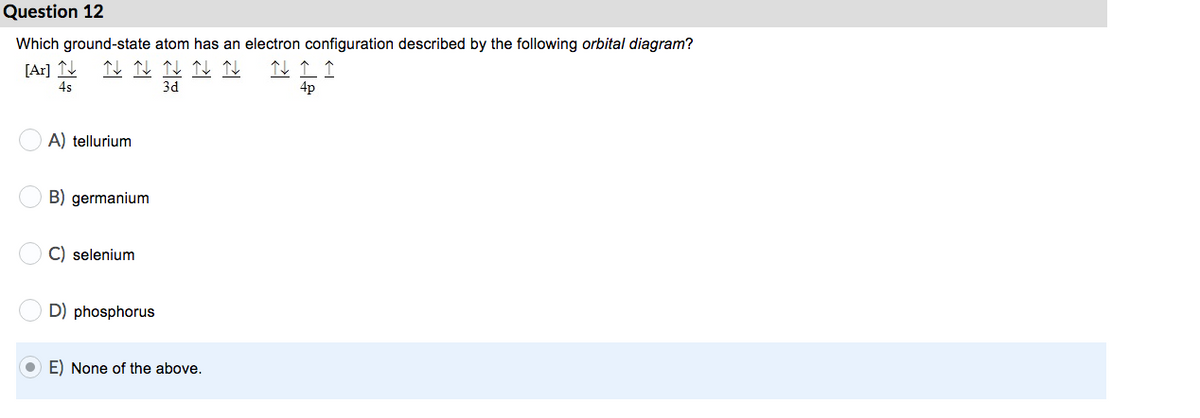
How to Write the Electron Configuration for Germanium (Ge ... A step-by-step description of how to write the electron configuration for Germanium (Ge).In order to write the Ge electron configuration we first need to kno... 41.THE PERIODIC TABLE – s,p,d,f ... - Madoverchemistry.com 14.3.2017 · e.g. energy of 4s orbital < energy of 3d orbital. (refer to the energy level diagram of orbitals in post 28).So, electrons enter 4s orbital first and 3d orbital later. [The order of filling of electrons in orbitals is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d .] Chemistry Chapter 7 Flashcards & Practice Test - Quizlet The orbital diagram for a ground-state nitrogen atom is A) A B) B C) C D) D see chart. A. The orbital diagram for a ground-state oxygen atom is ... Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these. C) selenium. Lone pair - Wikipedia In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pair.Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure.Electron pairs are therefore considered lone pairs if two electrons are paired but are …
Chemistry Chapter 7 Flashcards & Practice Test - Quizlet B) f orbital Basically, when l = 0 the is one orbital and it is called an s-orbital. It can hold 2 electrons total. When l = 1, the orbitals are called p-orbitals and there are 3 of them. Each of the individual p-orbitals can hold 2 electrons each. This gives us a total of 6 electrons that can go into the 3 p-orbitals. Electron configuration for Germanium (element 32). Orbital ... Ge (Germanium) is an element with position number 32 in the periodic table. Located in the IV period. Melting point: 937.4 ℃. Density: 5.32 g/cm 3 . Electronic configuration of the Germanium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 2. Electron Configuration Questions and Answers | Study.com Draw the orbital electron filling diagram, using the shortcut of (noble gas) to represent core electrons and up/down arrows to indicate all other electrons, for the … What is the orbital diagram for GE? 2022 - Question & Answers What is the orbital diagram for GE? Posted on January 19, 2022 by admin. Where is germanium found on Earth? We know germanium isn't a flower, but it's slightly harder to say just what it is. Most elements are either metals or nonmetals. Germanium falls in the same group as carbon and silicon, but also as tin and lead.
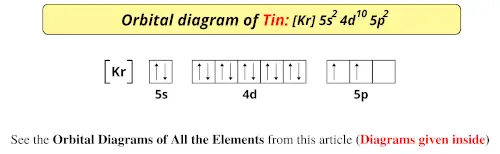
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40:
Silicon Orbital Diagram - ViralListClub.com Orbital diagrams are a visual way to show where the electrons are located within an atom. The remaining two electrons are placed in the 3p orbital. Carbon is above it. Orbital Diagram electron configuration and the noble gas notation for a silicon Si atom. The Aufbau principle the Pau. Germanium atomic orbital and chemical bonding information.
Periodic Table of Elements: Germanium - Ge ... Germaine is a hazardous substance UN2192 which is classified as a poisonous gas (2.3). It is also a flammable gas (2.1). Other compounds include: Germanium dichloride Ge Cl 2, Germanium dioxide GeO 2, Germanium tetrachloride GeCl 4 this is very irritating to eyes and membranes. Germanium Menu. Germanium Page One. Overview of Germanium
Germanium Orbital Diagram - schematron.org Germanium Orbital Diagram. Electron Configurations and Orbital Diagrams KEY. Draw orbital diagrams for the following elements: 1. phosphorus. ↑↓. ↑↓ 4. germanium. ↑↓. ↑↓. Mendeleev's Predicted Properties of Germanium ("eka. Silicon") and Its Use the orbital diagram to find the third and eighth electrons. PROBLEM: Write a set ...
37 orbital diagram for ge - Diagram Online Source Germanium Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
How many p orbitals does germanium have? 2022 - Question ... What is the orbital configuration of germanium? Electronic Configuration of Ge (Germanium) Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2 or it can be written as [Ar] 3d10 4s2 4p2. It has 2 electrons in K - shell, 8 electrons in L - shell, 18 electrons in M - shell and 4 electrons in its outermost shell N.
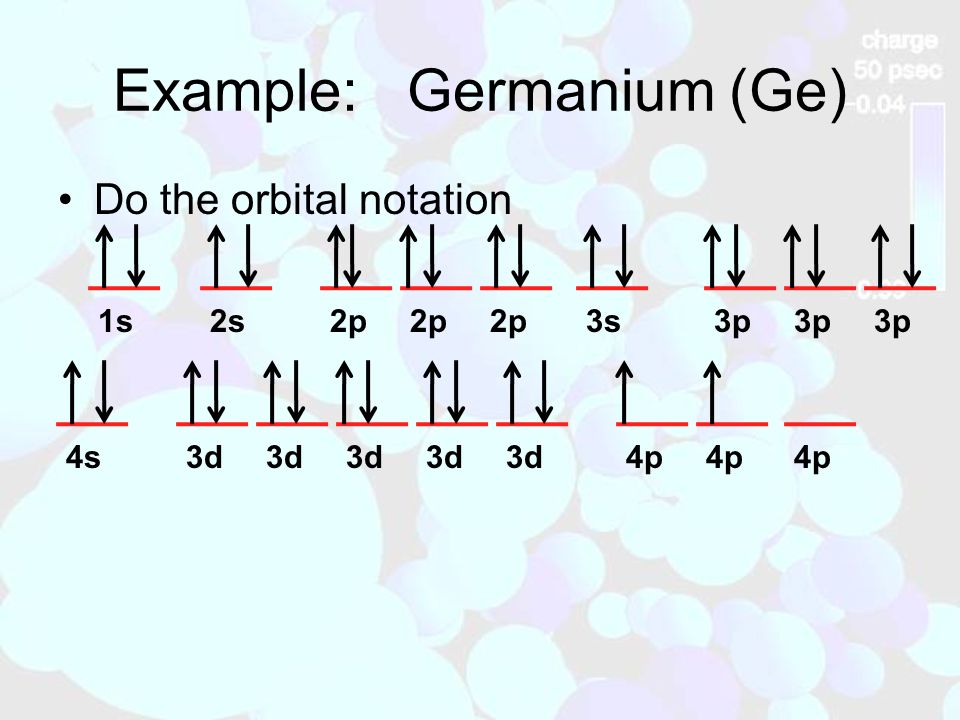
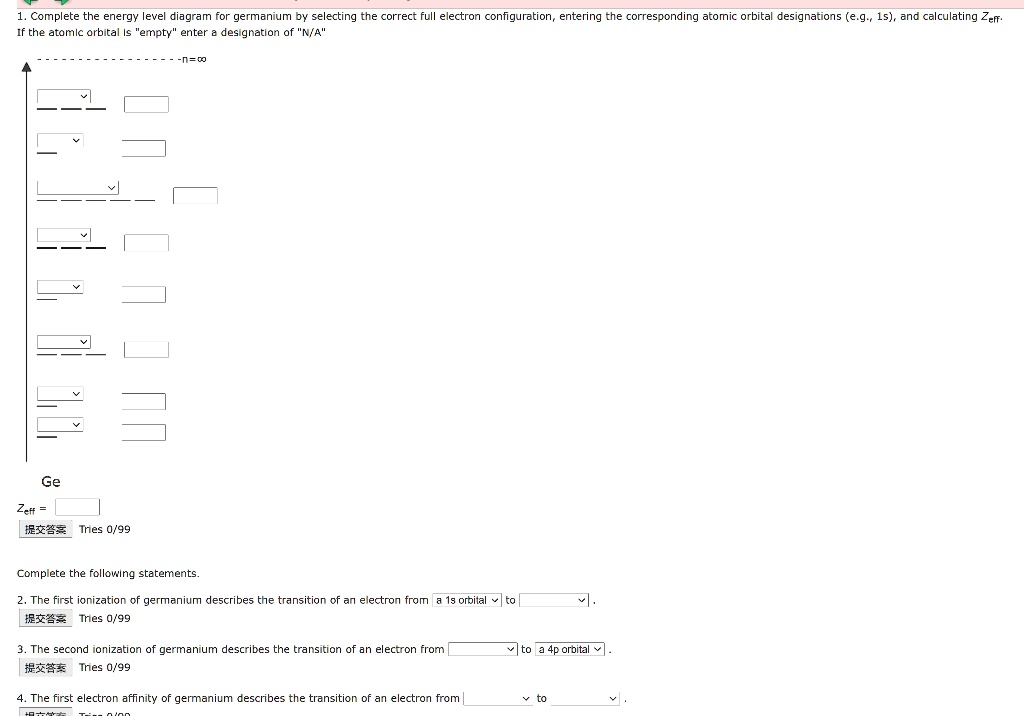
Solved 9: Do the complete orbital diagram for germanium at ... Science. Chemistry. Chemistry questions and answers. 9: Do the complete orbital diagram for germanium at the bottom of the page. From it, answer all of the following questions. a: # of occupied shells b: # of occupied subshells c: # of occupied orbitals d: # of filled shells e: # of filled subshells f: # of filled orbitals.
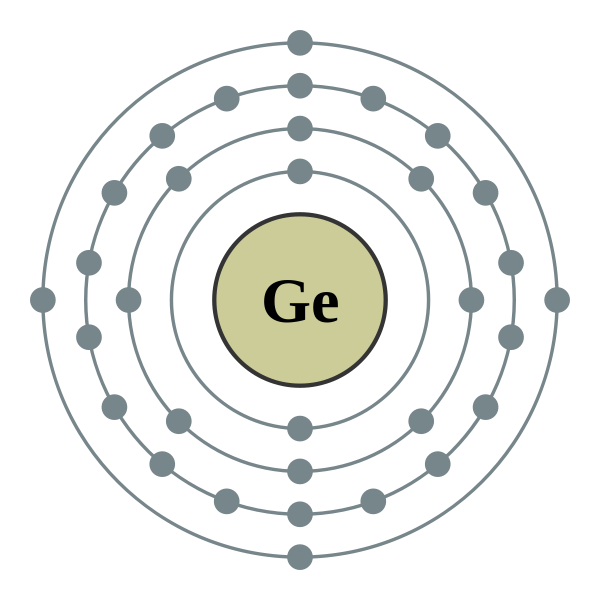
Chem4Kids.com: Germanium: Orbital and Bonding Info The orbital structure for germanium is 2-8-18-4. You can understand why there are similarities to silicon and tin when you see their orbital structures are 2-8-4 and 2-8-18-18-4 respectively. Watch for the 4. Because it has traits of elements like silicon and tin (just underneath), it is used by industry as a semiconductor material.
Iron(Fe) electron configuration and orbital diagram Iron ion (Fe 2+ ,Fe 3+) electron configuration. Ground state electron configuration of iron (Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons. In this case, the valence electrons of iron are eight.
What is the quantum orbital notation of germanium? - Answers Electron Notation is: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 Noble Gas notation is: [Ar] 4s2 3d10 4p2 Orbital Notation would be with the up & down arrow in boxes for each orbital.
Chapter 7: Quantum Theory and the Electronic Structure of ... 13. The orbital diagram for a ground-state oxygen atom is. 14. The orbital diagram for a ground state carbon atom is. 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these. 16.
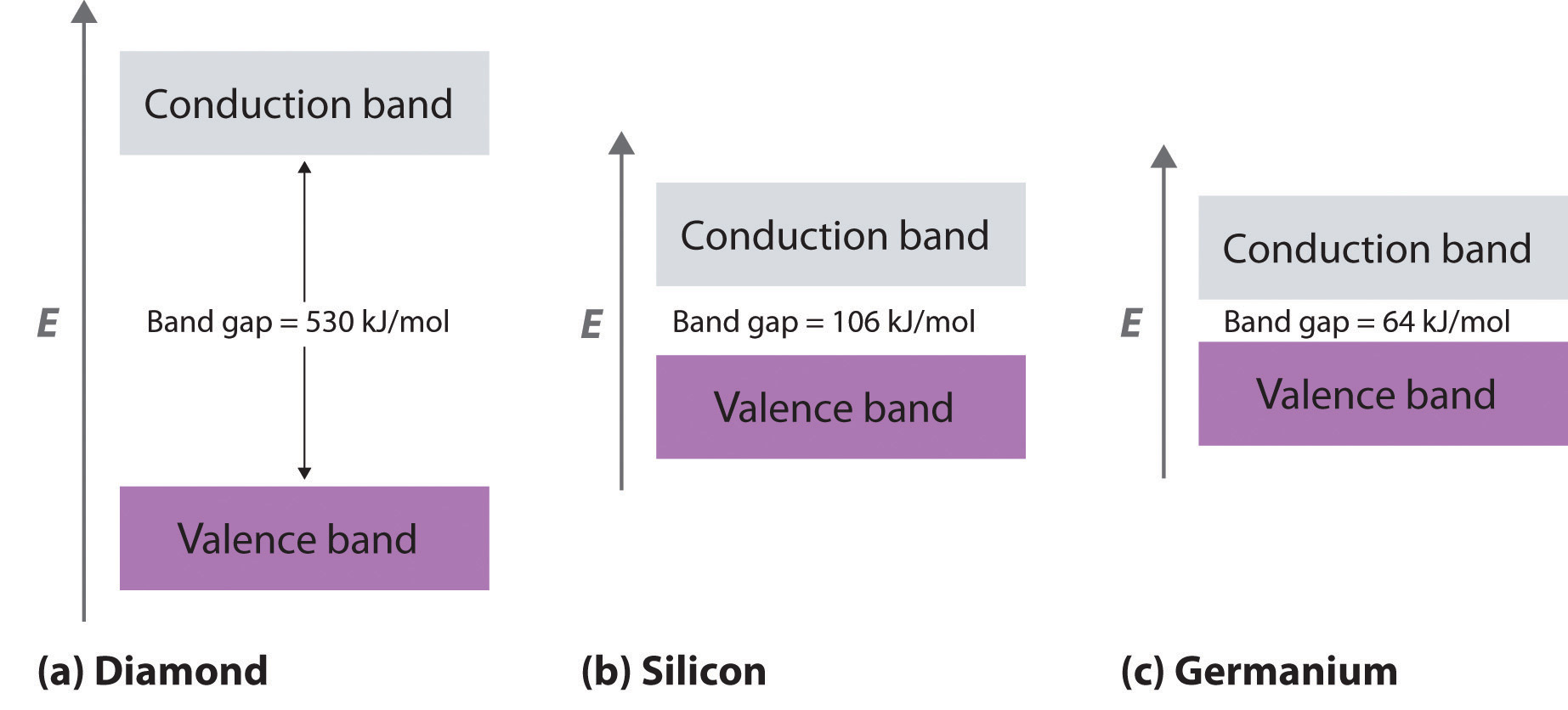
Dielectrics - The Physics Hypertextbook Discussion the basic idea. Dielectrics are insulators, plain and simple. The two words refer to the same class of materials, but are of different origin and are used preferentially in different contexts.
What is the orbital diagram for germanium? - Answers The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7.
Germanium(Ge) electron configuration and orbital diagram Germanium (Ge) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
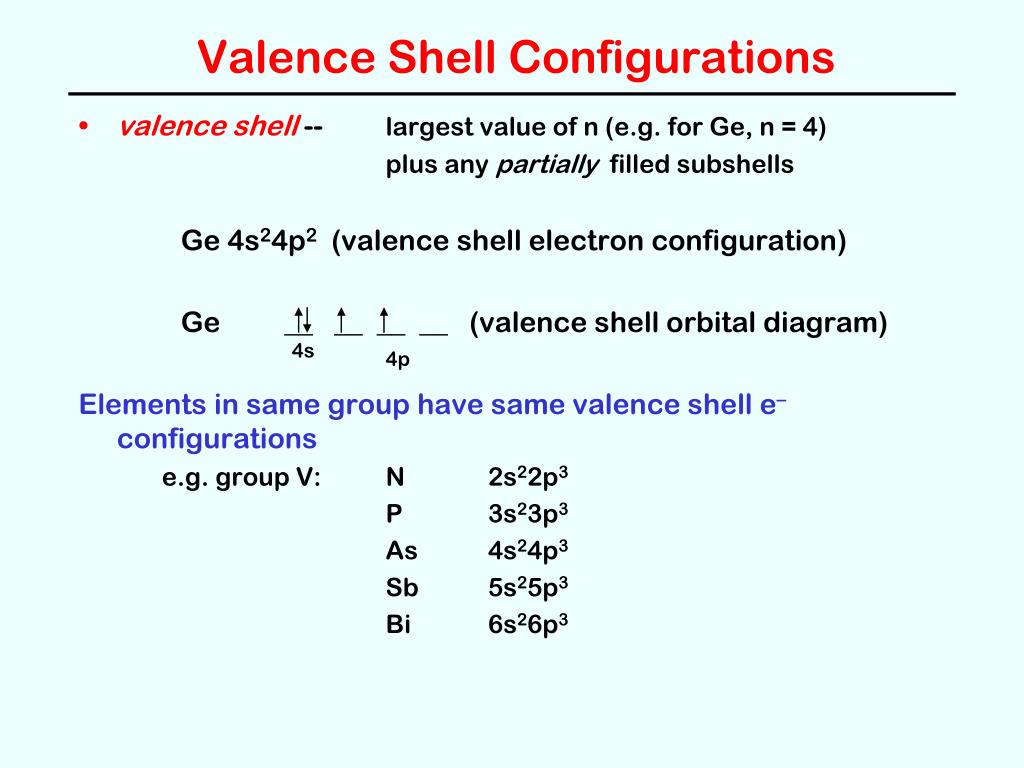
The inner orbital holds up to 2 electrons. Valence Shell ... 9.3.2022 · Figure1 shows sample images from AffectNet and their valence and arousal annotations. * Jan 26, 2021 · Sulfur Electron Configuration (S) with Orbital Diagram. 2. 64 km) How far is it from Valence to Example of Ionic Strength calculation – Example 2.
Orbital Diagram For Germanium - schematron.org orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2. Orbital Diagram. 1s. ↿⇂. 2s.
WebElements Periodic Table » Germanium » properties of ... Compound properties. Element reactions. Germanium atoms have 32 electrons and the shell structure is 2.8.18.4. The ground state electron configuration of ground state gaseous neutral germanium is [ Ar ]. 3d10. 4s2. 4p2 and the term symbol is 3P0. Schematic electronic configuration of germanium. The Kossel shell structure of germanium.
How many electrons are in each shell including 3p orbitals 19.1.2021 · The atom’s electrons exist in atomic orbitals, and we can identify the configuration of each of them using a set of precise guidelines and measures. The Bohr model, named after Neils Bohr, is a one-dimensional model that illustrates the electron’s composition and distribution in the atoms. While you need more information to determine the configuration, … How many …
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
Germanium (Ge) - ChemicalAid Germanium (Ge) has an atomic mass of 32. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.





















0 Response to "40 orbital diagram for germanium"
Post a Comment