41 d orbital energy level diagram
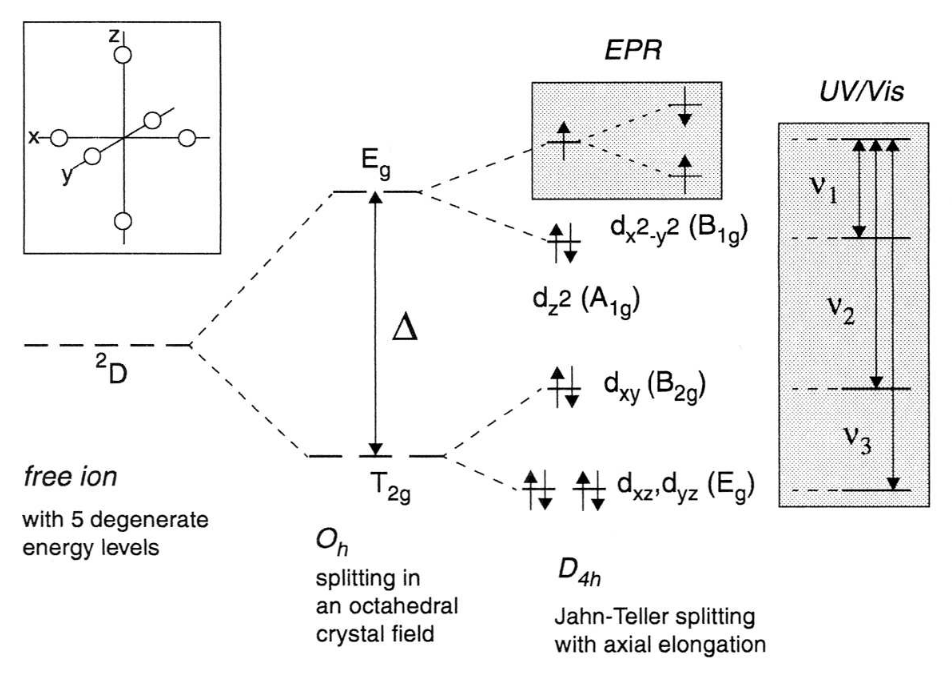
Molecular Orbital Theory: Energy level diagram for ... Molecular Orbital Theory: Energy level diagram for molecular orbitals. Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals. PDF Effect of Distortion on the d-Orbital Energy Levels Figure 46. The splitting pattern of d-orbital energy levels of d3-metal complexes in tetragonal distortion. 2. Rhombic distortion: The unequal amount of elongation or compression along two four-fold axes of rotation in octahedral complexes produces rhombic distortions. The common examples of rhombic distortion are high-
S P D F orbitals Explained - 4 Quantum Numbers, Electron ... This video explains s, p, d, and f orbitals, sublevels, and their shapes. It discusses the 4 quantum numbers n, l, ml, and ms. n represents the energy leve...
D orbital energy level diagram
s,p,d,f Orbitals - Chemistry | Socratic All levels except the first have p orbitals. d ORBITALS. In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz). At ... Lecture 3 Bonding Stable electronic configurations: MO Energy Level Diagrams Reviewed ... Ligand (σ- and π*) orbitals and metal d-orbitals are shown.) Simplified MO energy ...37 pages What is d orbital splitting in octahedral complex ... Therefore, the d electrons closer to the ligands will have a higher energy than those further away, which results in the d orbitals splitting in energy. Which complex has the greatest d orbital splitting? Violet - Greatest splitting means more energy is required to move an electron from a lower energy d-orbital to a higher one.
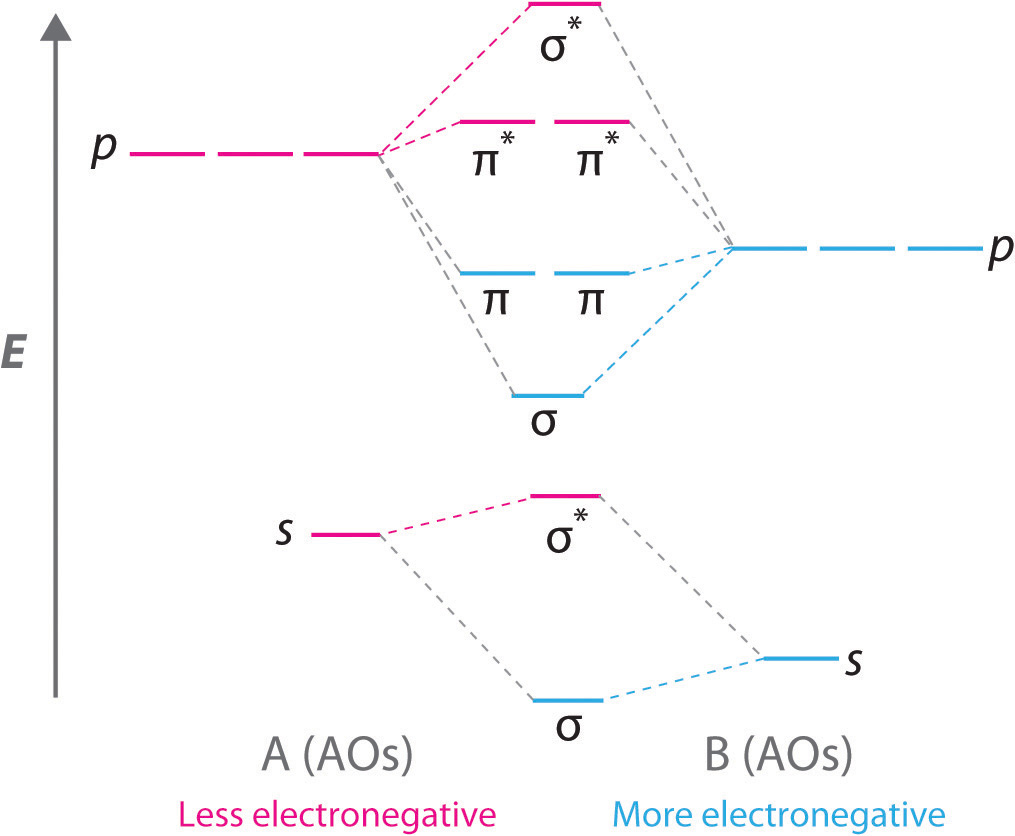
D orbital energy level diagram. Molecular orbitals energy level diagram - Big Chemical ... FIGURE 3.33 A typical d molecular orbital energy-level diagram for a heteronuclear diatomic molecule AB the relative contributions of the atomic orbitals to the molecular orbitals are represented by the relative sizes of the spheres and the horizontal position of the boxes. In this case, A is the more electronegative of the two elements. [Pg.246] Energy Of Orbitals: Concept, Factors, Observations, and ... Energy Level Diagrams: Some Important Observations. The energy level diagram can help us deduce that the energy of the subshells of a specific shell is not equal. The energies of 2s and 2p, for example, are different. The subshell with the lowest value of I has the lowest energy in a given shell. 2s (I = 0) has lower energy than 2p (I = 1) in the second shell. Energy Levels, Orbitals, Sub-Levels and Shells - vigglegiggle A P-Orbital in the second energy level is a 2p orbital ( 2p(x), 2p(y), 2p(z) ) A P-Orbital in the third energy level is a 3p orbital ( 3p(x), 3p(y), 3p(z) ) etc. In addition, the third and subsequent energy levels each contain five D-Orbitals, the fourth and subsequent energy levels contain seven F-Orbitals and so on. D3.3 Orbital Energy Level Diagrams - Chemistry 109 Fall 2021 An orbital energy level diagram (or just orbital diagram) shows the relative energies of orbitals and how electrons are distributed among orbitals within a subshell. In an orbital energy level diagram, individual orbitals are usually represented by horizontal lines whose vertical position conveys the qualitative relative energies of the orbitals.
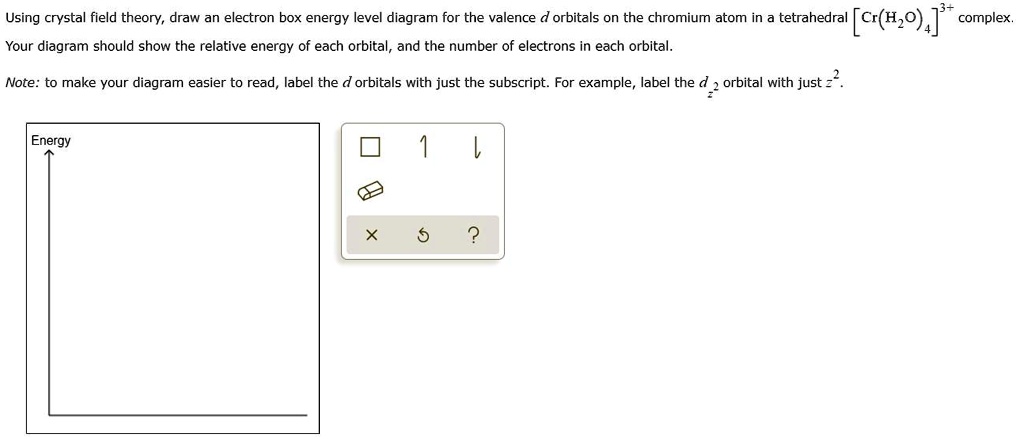
Phosphorus Orbital diagram, Electron configuration, and ... D orbital contains 5 boxes that can hold a maximum of 10 electrons. F orbital contains 7 boxes that can hold a maximum of 14 electrons. The orbital diagram will also be filled with the same order as described by the Aufbau principle. (1s < 2s < 2p < 3s……and so on.) What is the Orbital diagram for Phosphorus? Atomic orbital - Wikipedia For example, the orbital 1s (pronounced as the individual numbers and letters: "'one' 'ess'") is the lowest energy level (n = 1) and has an angular quantum number of ℓ = 0, denoted as s. Orbitals with ℓ = 1, 2 and 3 are denoted as p, d and f respectively. Solved Sketch the d-orbital energy level diagrams | Chegg.com Sketch the d-orbital energy level diagrams for [Fe(OH 2) 6] 3+ and [Fe(CN) 6] 3-. Use your diagrams to answer the questions. Use your diagrams to answer the questions. 1 a). Solved For each of the complexes (Co(NH3)6]3+ and ... - Chegg Transcribed image text: For each of the complexes (Co(NH3)6]3+ and [CoF6]3- (a) Draw an orbital energy level diagram that shows how the d-orbitals are split (ignore any Jahn-Teller distortion that might be present) and assign the appropriate labels (t2g, eg, e, or t2) to the d-orbitals. (b) Show how these orbitals are occupied by electrons and state whether each complex is paramagnetic or ...
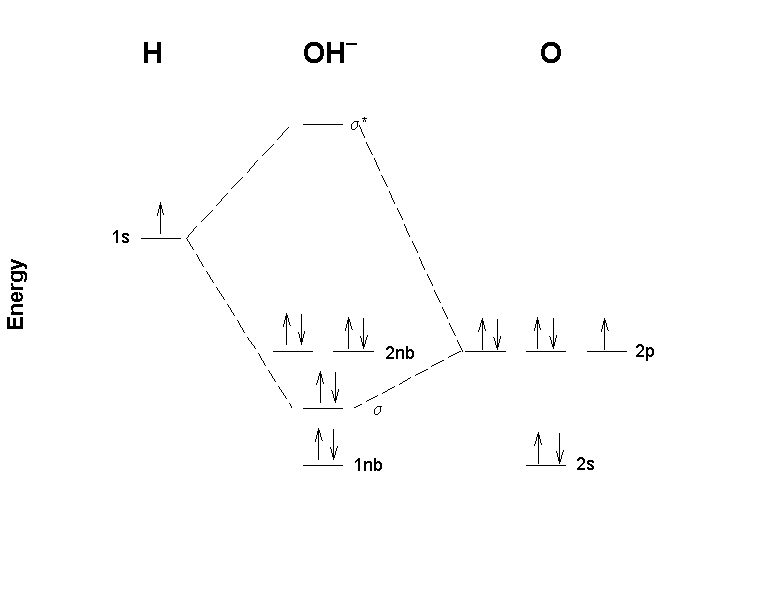
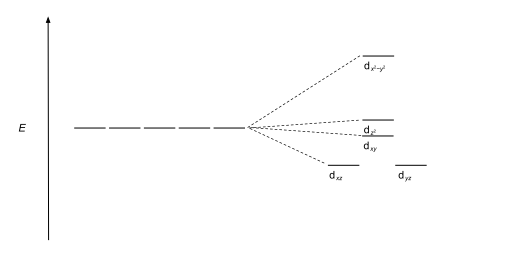
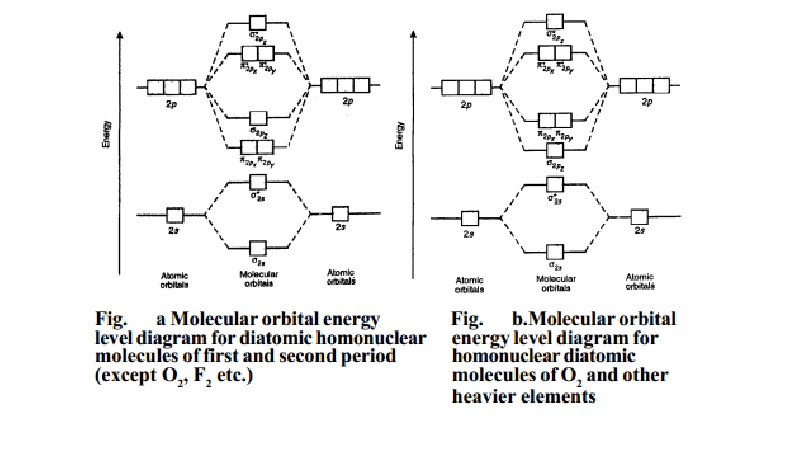
Orbital Diagram For Arsenic - schematron.org with the. d. orbital being one level lower than the energy level it is on. orbital diagram for arsenic see more ideas approximately from diagram electron configuration gallery & create your house design images related to pictures as well help you in locating the solution are seeking about itOrbital diagram for arsenic. PDF D-orbital splitting diagrams - University of California ... D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d Energy level diagram for Molecular orbitals - Chemical ... Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. 2) Stability of molecules in terms of bond order. PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
SOLVED:Sketch the d -orbital energy level diagrams for ... So the lower energy or bills are always filled first. And here we see that we have one unfairly electron. However, it in the other complex water is a relatively weak field splitting Lagan, so the electron, the energies of the orbital's are relatively similar. So as a result of hans rule, electrons are essentially added to each of the orbital's.
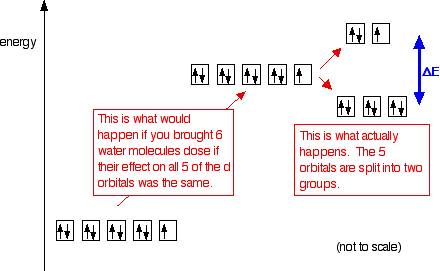
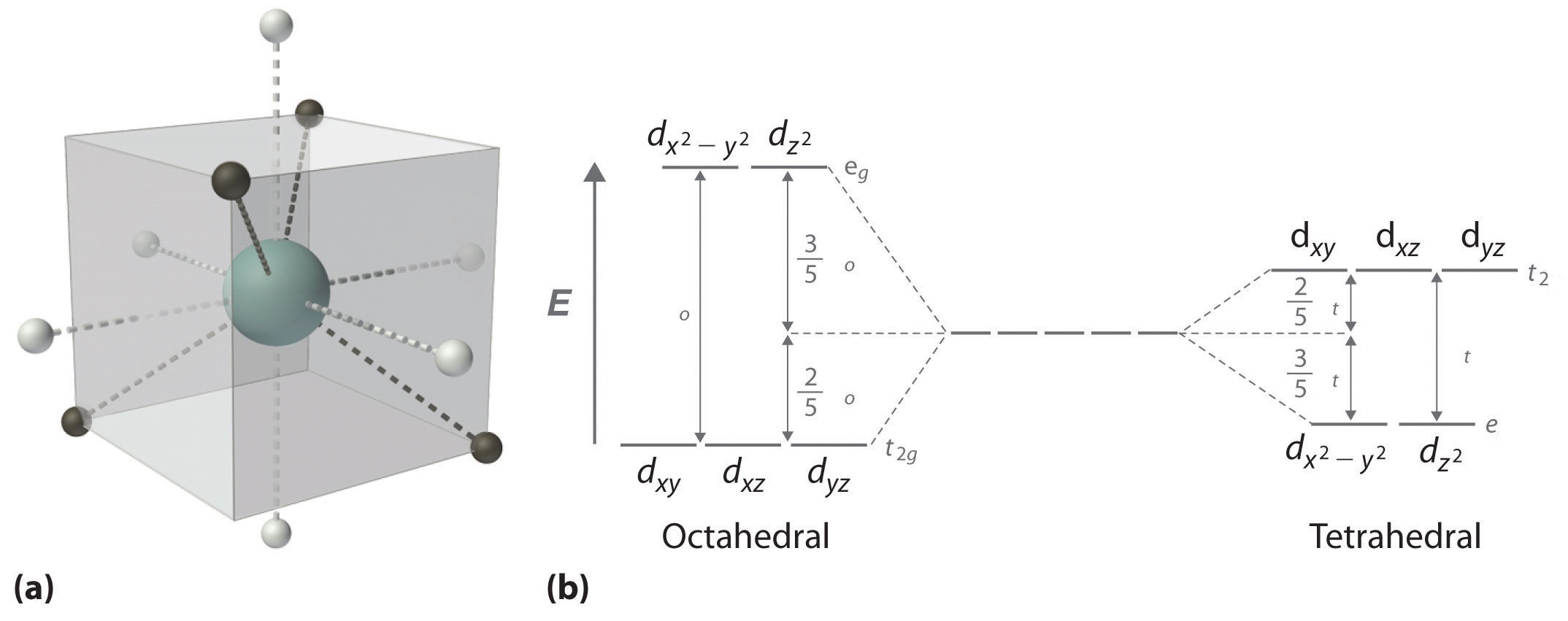
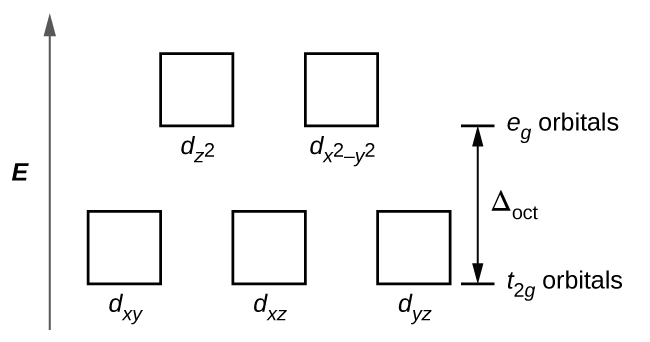
Crystal Field Theory - Chemistry LibreTexts Placing a charge of −1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies: the dx2−y2 and ...May 6, 2021 · Uploaded by Chuck WightCrystal Field Stabilization Energy · Introduction to Crystal Field...
Energy Level Diagram - Different Energy Shells Around the ... Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows: s ...
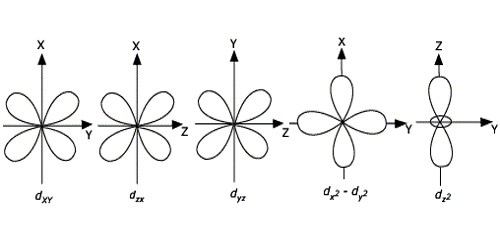
Orbitals Chemistry (Shapes of Atomic Orbitals) - Shape of ... Similar to s orbitals, size, and energy of p orbitals increases with an increase in the principal quantum number (4p > 3p > 2p). The Shape of d Orbitals. The magnetic orbital quantum number for d orbitals is given as (-2,-1,0, 1,2). Hence, we can say that there are five d-orbitals. These orbitals are designated as d xy, d yz, d xz, d x 2 -y 2 ...
Molecular Orbital Theory - Chemistry Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
Nitrogen(N) electron configuration and orbital diagram The electron configuration of all the elements can be done through orbital diagram. Electron configuration of nitrogen atom through orbital. Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by 'l'. The value of 'l' is from 0 to (n - 1).
Energy of Orbitals - Calculating the Energy Level, Solved ... The s-orbital has the azimuthal quantum number value of 0, the p-orbital has a value of 1, and the d-orbital has two and f-orbital 3. The orbital energy levels of these are varied, s-orbital having the lowest energy level and f-orbital being the highest, summed up as s < p < d < f.
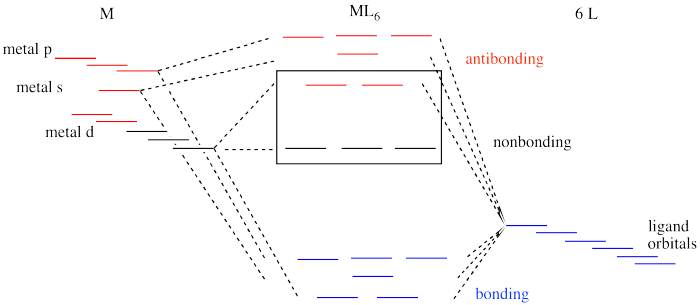
PDF Molecular Orbital Theory - Octahedral, Tetrahedral or ... The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation,
How to Do Orbital Diagrams - Sciencing The Aufbau principle tells you that the lowest-energy orbitals fill first, but the specific order isn't sequential in a way that's easy to memorize. See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n = 3 level only has s, p and d orbitals.
Question: Do We Draw The D Orbitals In Mo Diagrams ... Steps for Drawing an Orbital Diagram Label the arrow energy. The arrow shows a qualitative representation of increasing orbital energy. Write out the electron configuration to determine which orbitals are filled. Along the right side of the energy arrow, write each electron shell that is being filled. Does d orbitals form pi bond?
PDF Metal d orbitals in an Oh crystal field • The energy increase of the e g orbitals and the energy decrease of the t 2g orbitals must be balancedrelative to the energy of the hypotheticalsphericalfield(aka the barycenter).• The energy of each of the two orbitals of the e g set rises by +3/5 o (+6 Dq) while the energy of eachof the three t 2g orbitalsfallsby ‐2/5 o(‐4Dq). • Thisresults inno netenergy changefor the system:
Copper(Cu) electron configuration and orbital diagram The Aufbau method is to do electron configuration through the sub-energy level. These sub-orbitals are expressed by 'l'. The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. These orbitals are named s, p, d, f.
Energy Levels, Orbital Diagrams, Electron Config, Noble ... Energy Levels, Orbital Diagrams, Electron Config, Noble Gas - Google Slides. Energy Levels. We know, atoms have energy levels. The periodic table shows us energy levels 1 - 7. Energy Levels. By default electrons are found in the lowest energy level possible, close to the nucleus. This is called the ground state.
What is d orbital splitting in octahedral complex ... Therefore, the d electrons closer to the ligands will have a higher energy than those further away, which results in the d orbitals splitting in energy. Which complex has the greatest d orbital splitting? Violet - Greatest splitting means more energy is required to move an electron from a lower energy d-orbital to a higher one.
Lecture 3 Bonding Stable electronic configurations: MO Energy Level Diagrams Reviewed ... Ligand (σ- and π*) orbitals and metal d-orbitals are shown.) Simplified MO energy ...37 pages
s,p,d,f Orbitals - Chemistry | Socratic All levels except the first have p orbitals. d ORBITALS. In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz). At ...















![d-orbital energy levels in planar [M II F 4 ] 2− , [M II (NH ...](https://pubs.rsc.org/image/article/2020/DT/d0dt02022b/d0dt02022b-f1_hi-res.gif)


0 Response to "41 d orbital energy level diagram"
Post a Comment