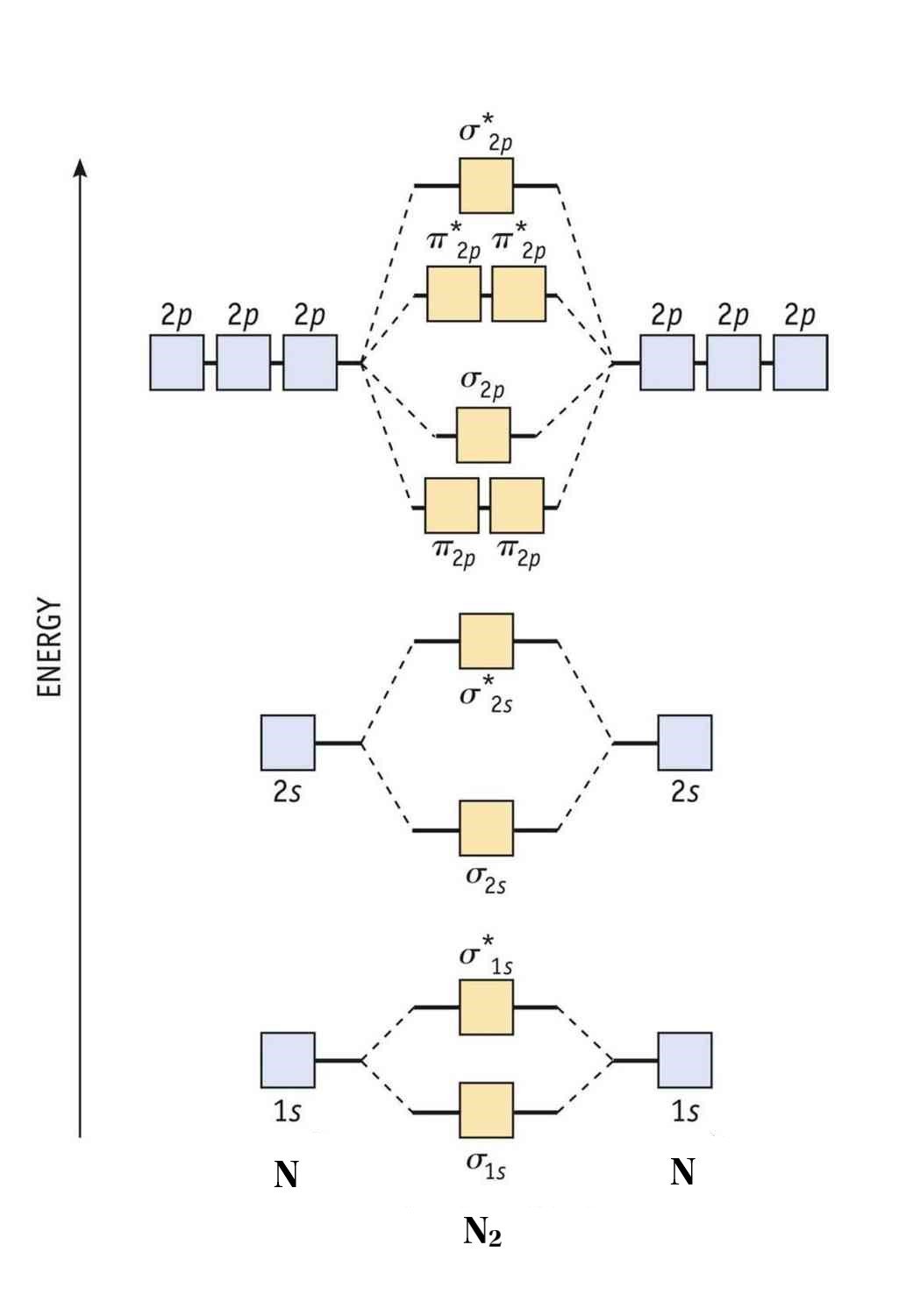
41 mo diagram for li2
Answered: Use the MO diagram (below) to calculate… | bartleby Transcribed Image Text: Use the MO diagram (below) to calculate the bond order for l2. Op - Op 1 2 3 4 6. - Os 8 +/- x 100 LO M.O. Diagram for B2 - CHEMISTRY COMMUNITY As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond.
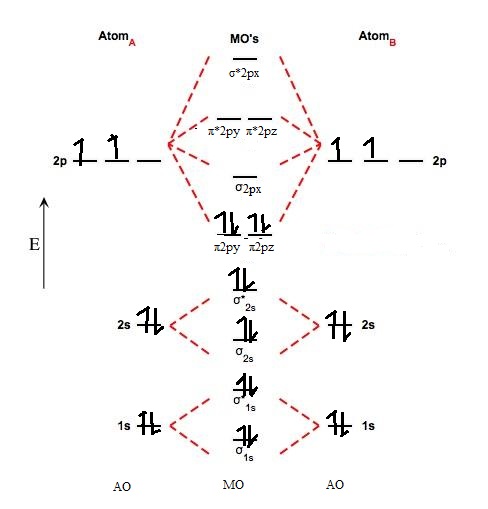
Molecular Orbital Diagrams of Diatomic Molecules - Chem Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.In this theory, each molecule has a set of molecular orbitals. Objectives: Practice energy diagrams for molecular orbital theory.
Mo diagram for li2
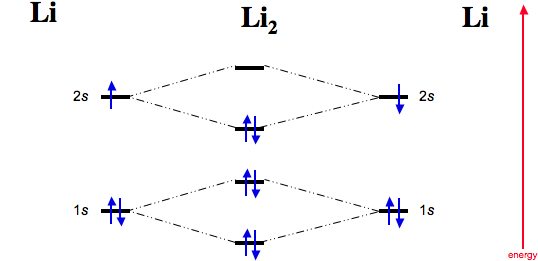
41 li2+ molecular orbital diagram - Wiring Diagrams Manual Question: Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form ... non102glucose 😋obese Chamberlain, 2008 WL 4862717, at *2 (E.D. Mo. Nov. 7, 2008) (quoting Kikumura v. Hurley, 242 F.3d 950, 955 (10th Cir. 2001)).|Plaintiff alleges irreparable harm in that if Defendant terminates their contract, it will not be able to remain in business. Also, Plaintiff argues that its customers will be adversely affected and will not be able to obtain their needed diabetic strips. Plaintiff has ... Molecular Orbital Diagram For Li2 - schematron.org Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital. This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be .
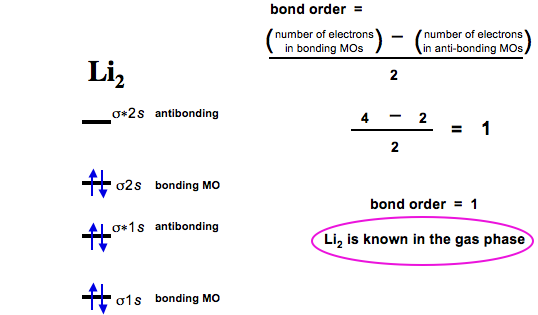
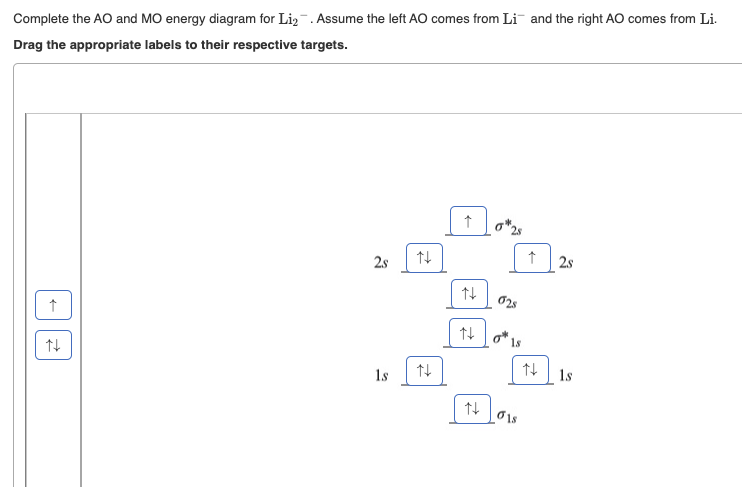
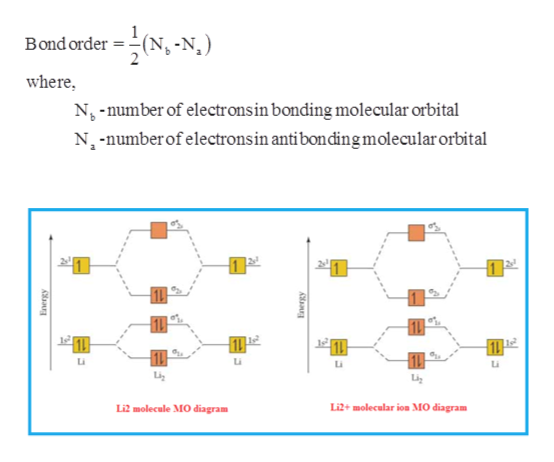
Mo diagram for li2. Solved 1)Complete the atomic orbital (AO) and ... - Chegg 1)Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li2+ and Li2-. Best Answer. This is the best answer based on feedback and ratings. 81% (21 ratings) Li2- Molecular Orbital Diagram Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. Uncovering electrocatalytic conversion mechanisms from ... This work draws a comprehensive diagram for Li 2 S 2 RR mechanism, and proves a fast and spontaneous conversion that happens before the crystallization of Li 2 S can be achieved with the help of highly-active electrocatalysis. It provides theoretical guidance for designing the Li–S battery with high energy density. More importantly, it proves the CHE approach can be … Molecular Orbital Diagram For Li2 - Wiring Diagrams MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1.
Use the molecular orbital diagram shown to determine which ... Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons. Essentials of Physical Chemistry by B.S ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of Flour Mill Rye [4MH368] Search: Rye Flour Mill. What is Rye Flour Mill. Every flour has its own unique properties. Sourdough Rye using your flour and some crushed organic caraway seeds has lifted my Sourdough Rye to a new level!! Li2 Mo Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and NO. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2.
PDF Is li2 4- paramagnetic or diamagnetic - Weebly Monday, September 14, 2011, 10:44 Li2 has only two electrons. When drawing MO diagrams, both must be placed in a sigma 2s join trajectory. Both are paired, so they are antimagnetic. Ignore what the book did to get bond orders. If you use Z<8 to draw a MO diagram of Li2, you need to get the same answer and bond order. MOT | Molecular Orbital Energy level Diagram for Li2, Li2 ... This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o... MO Diagrams - GitHub Pages #3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron. Mechanism and Free-Energy Landscape of Peptide Bond ... 11.02.2022 · The amino acid condensation reaction on a heterogeneous mineral surface has been regarded as one of the important pathways for peptide bond formation. In this work, the mechanism of peptide bond formation over a silica surface in an aqueous environment is studied using ab initio molecular dynamics calculations coupled with enhanced sampling methods such …
Answered: Use the MO diagrams to calculate the… | bartleby Use the MO diagrams to calculate the bond order for Lig+ and Lig. Express the bond order for Li2* followed by the bond order for Li2 separated by a comma.
What Is The Bond Order Of Li2 The molecule Li2 is a stable molecule in the gas phase, with a bond order of one. Is Li2 − Li2 − paramagnetic or diamagnetic?, Li2 only has 2 electrons. If you draw the MO diagram, they should both be in the sigma 2s bonding orbital. They are both paired, so it is diamagnetic.
Draw the molecular orbital (MO) energy dia... | Clutch Prep Problem: Draw the molecular orbital (MO) energy diagram for Li2+. FREE Expert Solution Show answer Answer: 87% (350 ratings) Sign up for free to keep watching this solution Sign up for free. 609,953. students enrolled. 97%. improved grades. 2,784. minutes of videos.
What is the bonding order of Li2? - Quora above, you can see MO diagram for Li2 molcular orbitals. 6 regular electrons of neutral molecule taked place in sigma1, simga*1, and two seprated electrons in two Pi1 orbitals. one less electron goes In pi1, reduces bond order, therefore stability of bindings. So Li2- is a more stable by molecular orbital study, but a negtive charge on a tiny ...
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Is li2 - paramagnetic or diamagnetic? - TreeHozz.com The molecule Li2 is a stable molecule in the gas phase, with a bond order of one. Bond Order=2(bonding electrons)−0(anti−bonding e−)2=1. Beside above, is li2 stable or unstable? Li2+ is more stable than Li2− because Li2− has more numbers of antibonding electrons. The species in which the N atom is in a state of sp hybridization is: NO.
What Is The Bond Order Of Li2− The molecule Li2 is a stable molecule in the gas phase, with a bond order of one. Is Li2 − Li2 − paramagnetic or diamagnetic?, Li2 only has 2 electrons. If you draw the MO diagram, they should both be in the sigma 2s bonding orbital. They are both paired, so it is diamagnetic.
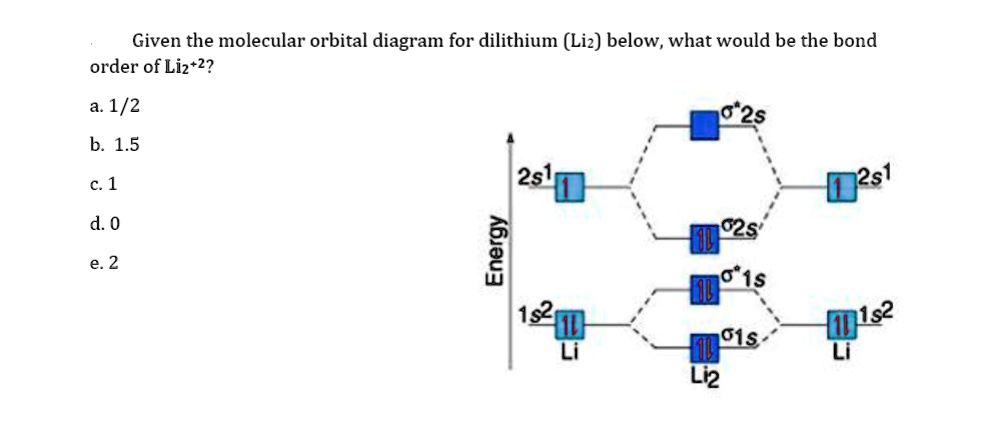
SOLVED:Draw an MO energy diagram and predict the bond ... Draw an MO energy diagram and predict the bond order of Li2+and Li2-. Do you expect these molecules to exist in the gas phase? Answer. See drawing. Since both have a bond order of $+\frac{1}{2},$ both $\mathrm{Li}_{2}^{+}$ and $\mathrm{Li}_{2}^{-}$ will exist in gas phase. ... Draw a molecular orbital energy level diagram for each of the ...
Solved Part A Use the drawing of the MO energy diagram to ... Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
Li2 Mo Diagram - schematron.org Molecular orbital (MO) theory describes the behavior of electrons in a be in a bonding orbital, we would predict the Li2 molecule to be stable. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = ().
Answer Key to the Exercises of Applied ... - Academia.edu Academia.edu is a platform for academics to share research papers.
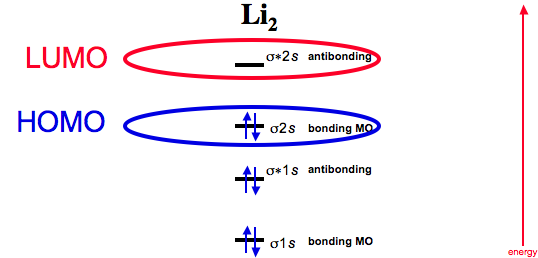
Dilithium - Wikipedia Dilithium, Li 2, is a strongly electrophilic, diatomic molecule comprising two lithium atoms covalently bonded together. Li 2 is known in the gas phase.It has a bond order of 1, an internuclear separation of 267.3 pm and a bond energy of 102 kJ/mol or 1.06eV in each bond. The electron configuration of Li 2 may be written as σ 2.. It has been observed that 1% (by mass) of lithium in the vapor ...
Molecular Orbital Diagram (MO Diagram) of Li2 - YouTube Full first shell:sigma-1s and sigma1s* are both full.sigma-2s is full; sigma-2s* is empty.Bond order 1 = single bond.Check me out:
MO diagram for Li2 - CHEMISTRY COMMUNITY MO diagram for Li2. Moderators: Chem_Mod, Chem_Admin. 2 posts • Page 1 of 1. Chem_Mod Posts: 21163 Joined: Thu Aug 04, 2011 8:53 pm Has upvoted: 1135 times. MO diagram for Li2. Post by Chem_Mod » Mon Nov 21, 2011 11:21 pm . Question: For molecules like Li2 where the p MO's aren't used and thus have no electrons, do we still have to draw them ...
Why #"Li"_2^+# is more stable than #"Li"_2# - Socratic.org Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding character strengthens the bond...
Which of the following is paramagnetic? (use the mole... Which of the following is paramagnetic? (use the molecular orbital diagram) a) Li 2. b) Be 2. c) B 2. d) C 2. e) N 2. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos.
40 molecular orbital diagram for he2+ - Diagram For You The antibonding orbital is empty. Thus, H2 is a stable molecule. Construct the molecular orbital diagram for he2 Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each.
Molecular Orbital Diagram For Li2 - schematron.org Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital. This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be .
non102glucose 😋obese Chamberlain, 2008 WL 4862717, at *2 (E.D. Mo. Nov. 7, 2008) (quoting Kikumura v. Hurley, 242 F.3d 950, 955 (10th Cir. 2001)).|Plaintiff alleges irreparable harm in that if Defendant terminates their contract, it will not be able to remain in business. Also, Plaintiff argues that its customers will be adversely affected and will not be able to obtain their needed diabetic strips. Plaintiff has ...
41 li2+ molecular orbital diagram - Wiring Diagrams Manual Question: Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form ...























0 Response to "41 mo diagram for li2"
Post a Comment