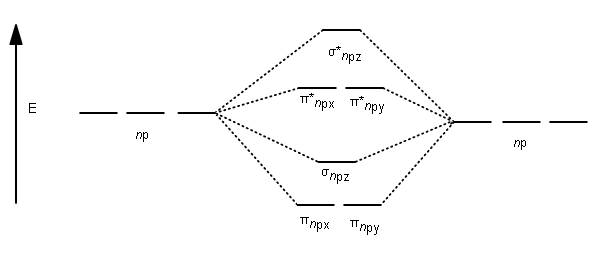
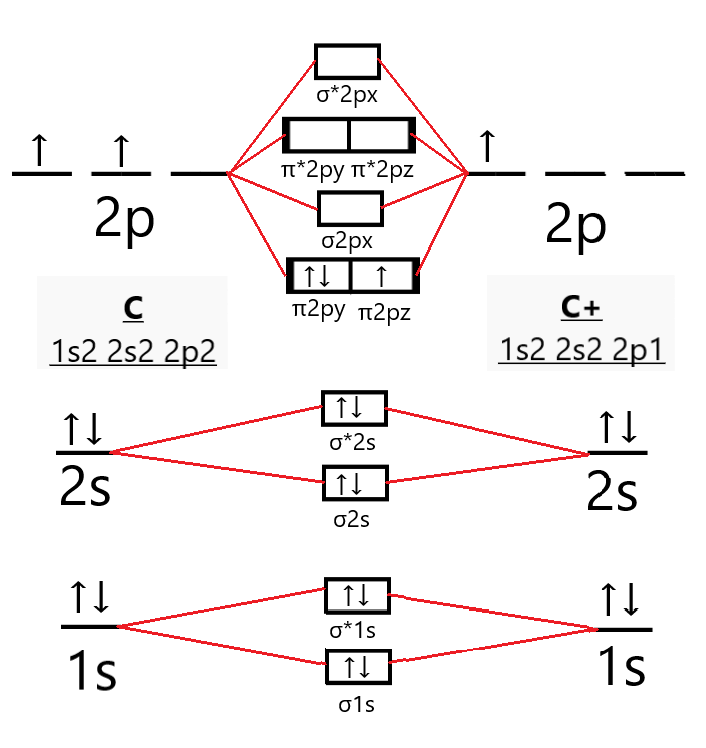
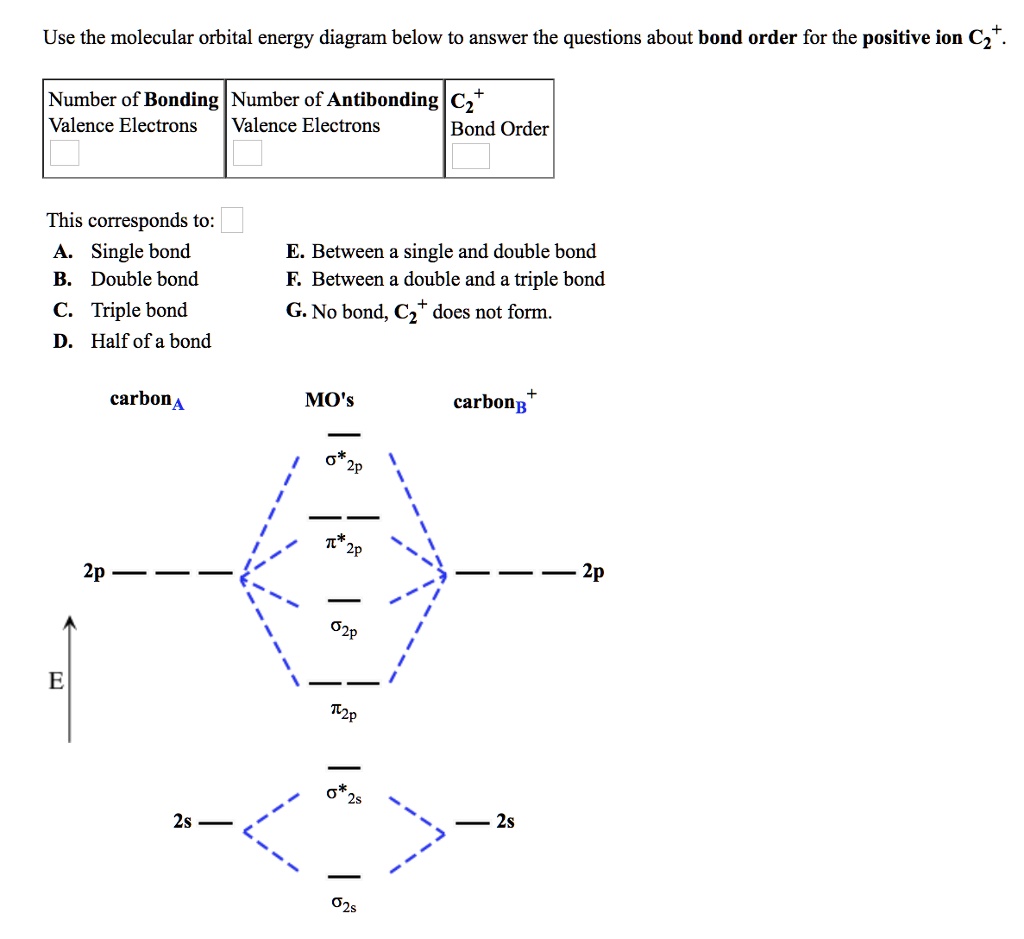
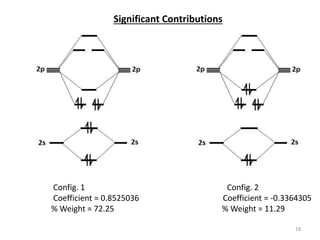
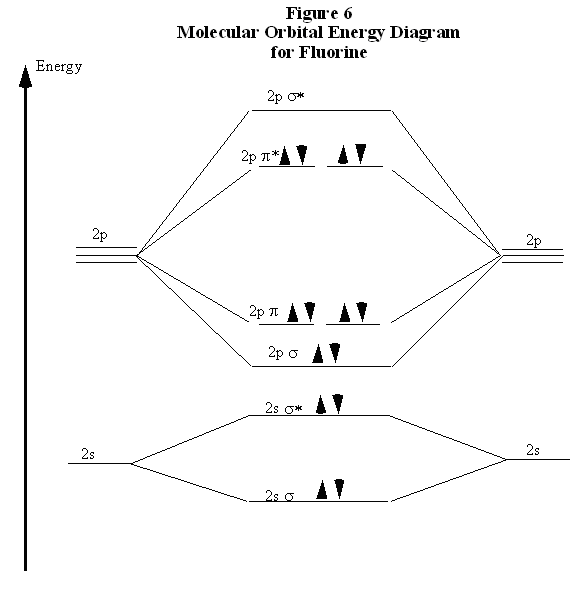
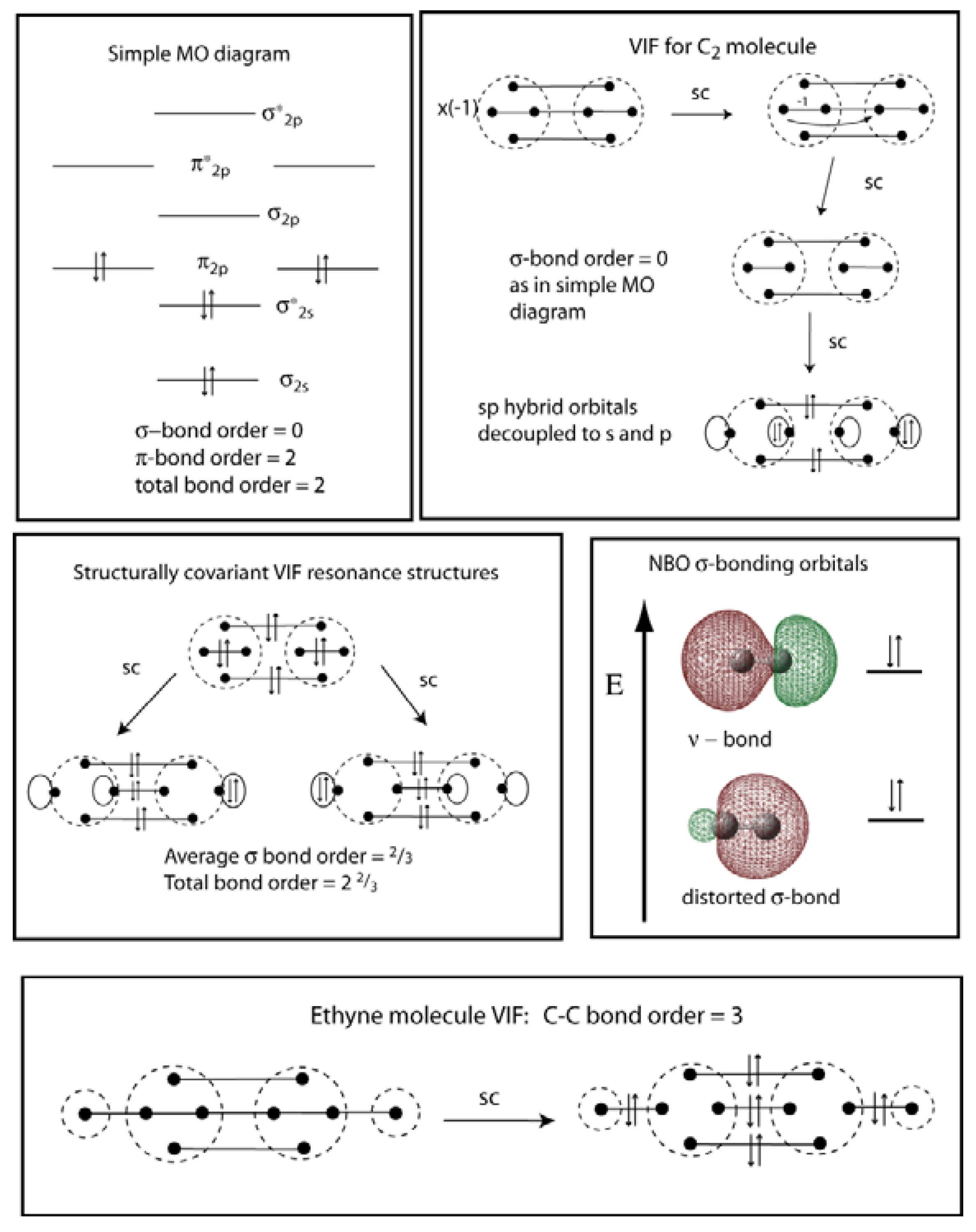
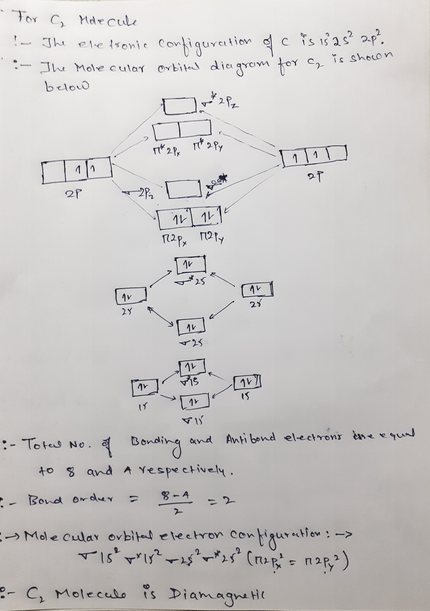
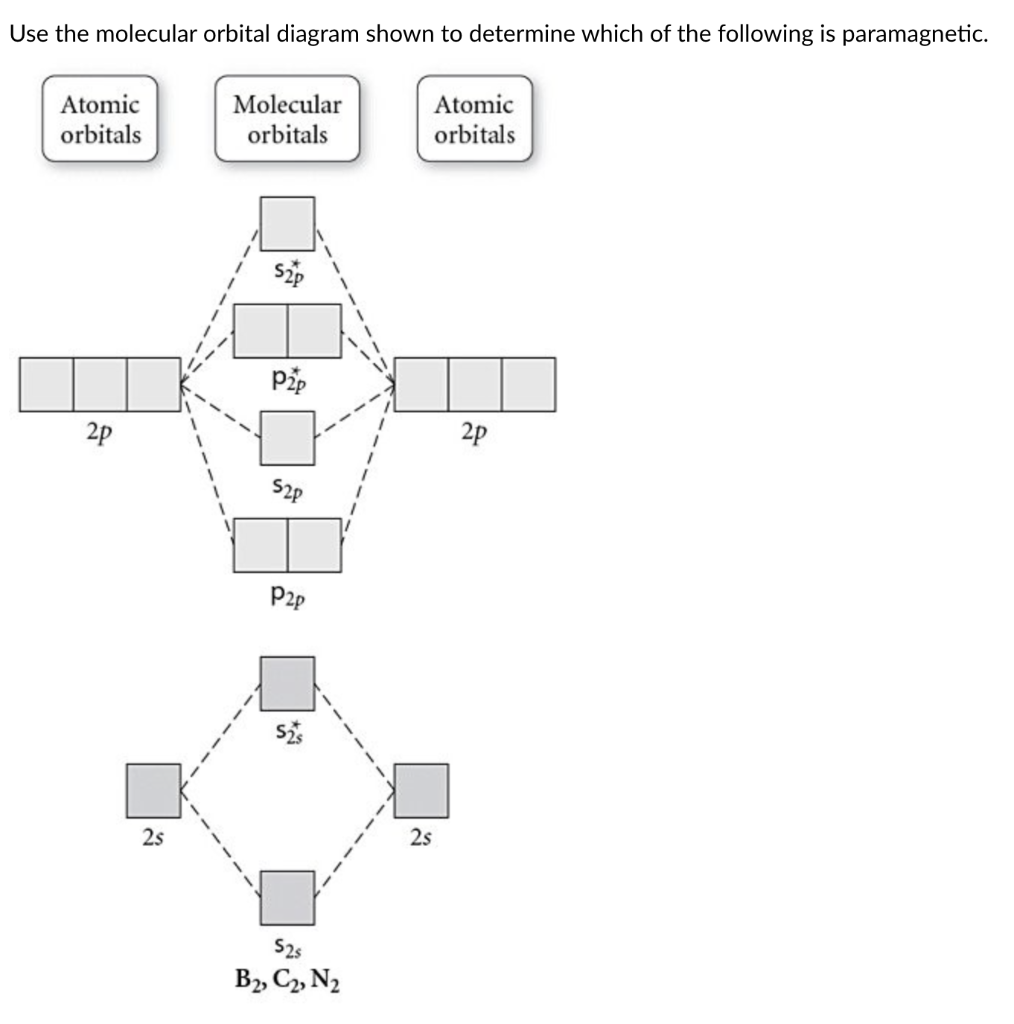
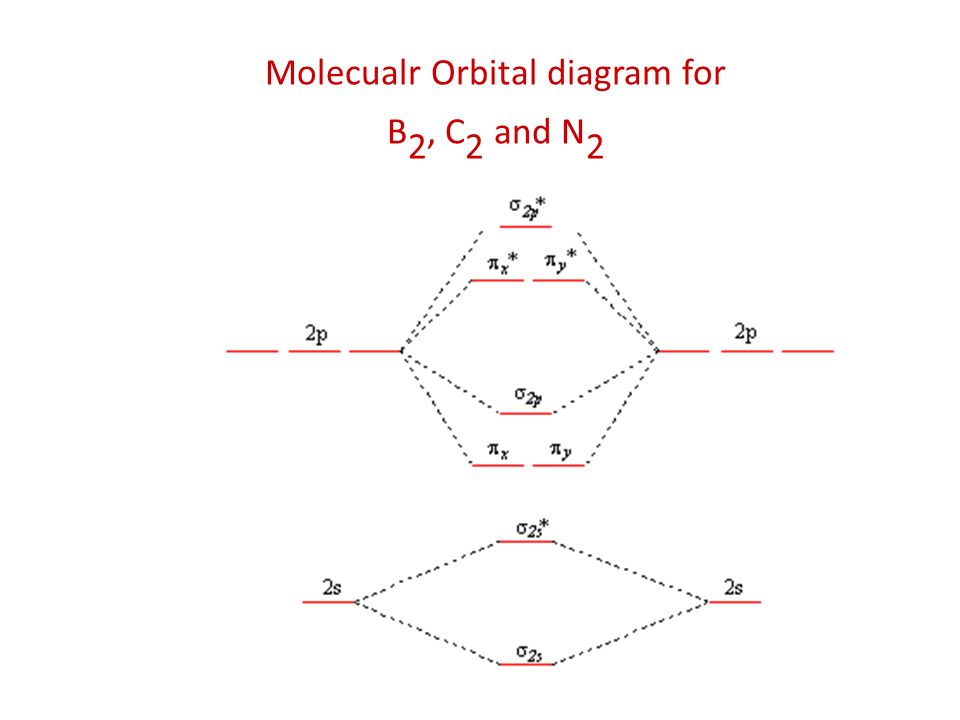
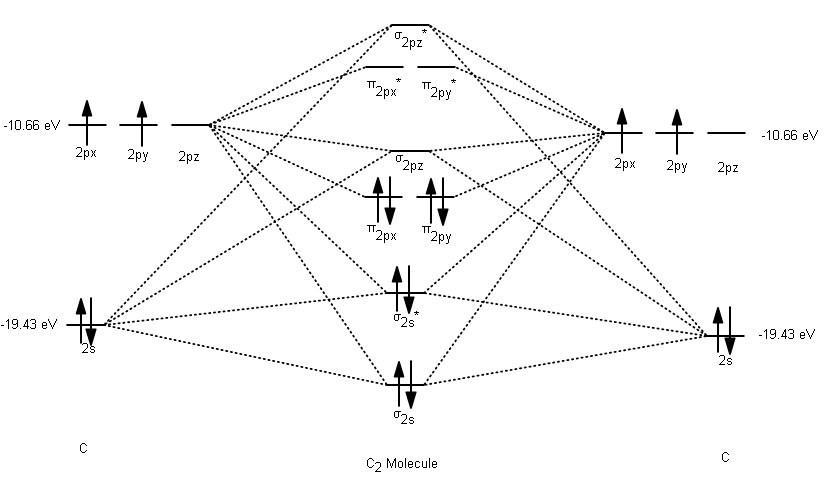
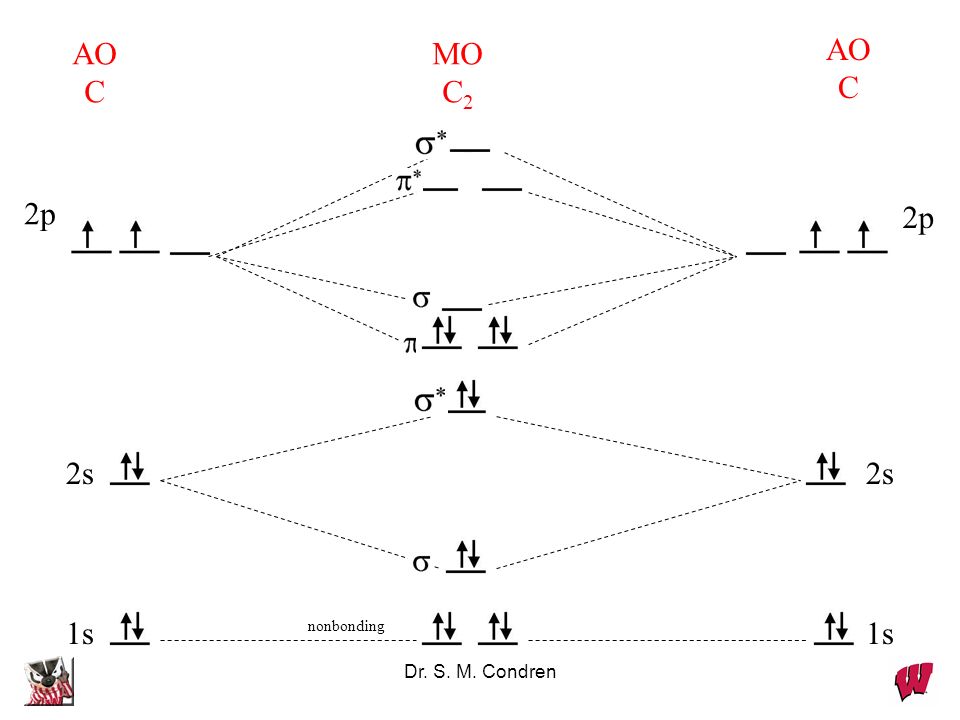
38 c2- molecular orbital diagram
(PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. . × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. ... ʻOumuamua - Wikipedia ʻOumuamua is small and not very luminous. It was not seen in STEREO HI-1A observations near its perihelion on 9 September 2017, limiting its brightness to approximately 13.5 mag. By the end of October, ʻOumuamua had already faded to about apparent magnitude 23, and in mid-December 2017, it was too faint and fast moving to be studied by even the largest ground …
Stereoisomers - Michigan State University The following diagram illustrates the change in potential energy that occurs with rotation about the C2–C3 bond. The model on the right is shown in conformation D, and by clicking on any of the colored data points on the potential energy curve, it will change to the conformer corresponding to that point. The full rotation will be displayed by ...

C2- molecular orbital diagram
Field-tunable toroidal moment and anomalous Hall effect in ... While H c2 remains nearly unchanged, H c1 increases rapidly with decreasing temperature from T N2 and reaches almost 9 T at 2 K (Fig. 2c). Indeed, the crossover between H c1 and H c2 should occur ... Stereoisomers - Michigan State University Stereoisomers. As defined in an earlier introductory section, isomers are different compounds that have the same molecular formula. When the group of atoms that make up the molecules of different isomers are bonded together in fundamentally different ways, we refer to such compounds as constitutional isomers.For example, in the case of the C 4 H 8 hydrocarbons, …
C2- molecular orbital diagram. Stereoisomers - Michigan State University Stereoisomers. As defined in an earlier introductory section, isomers are different compounds that have the same molecular formula. When the group of atoms that make up the molecules of different isomers are bonded together in fundamentally different ways, we refer to such compounds as constitutional isomers.For example, in the case of the C 4 H 8 hydrocarbons, … Field-tunable toroidal moment and anomalous Hall effect in ... While H c2 remains nearly unchanged, H c1 increases rapidly with decreasing temperature from T N2 and reaches almost 9 T at 2 K (Fig. 2c). Indeed, the crossover between H c1 and H c2 should occur ...











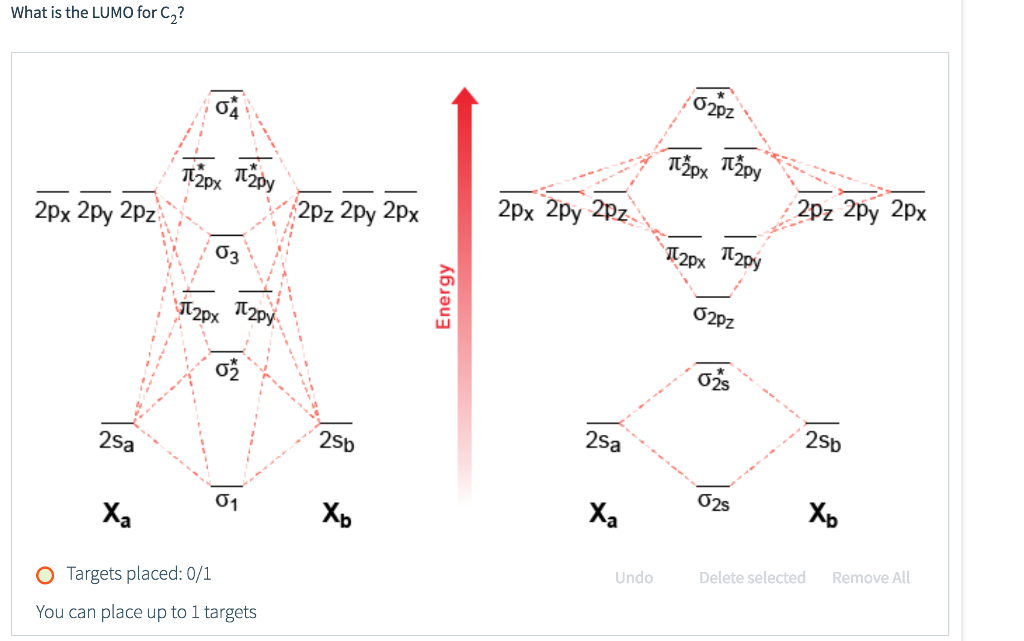


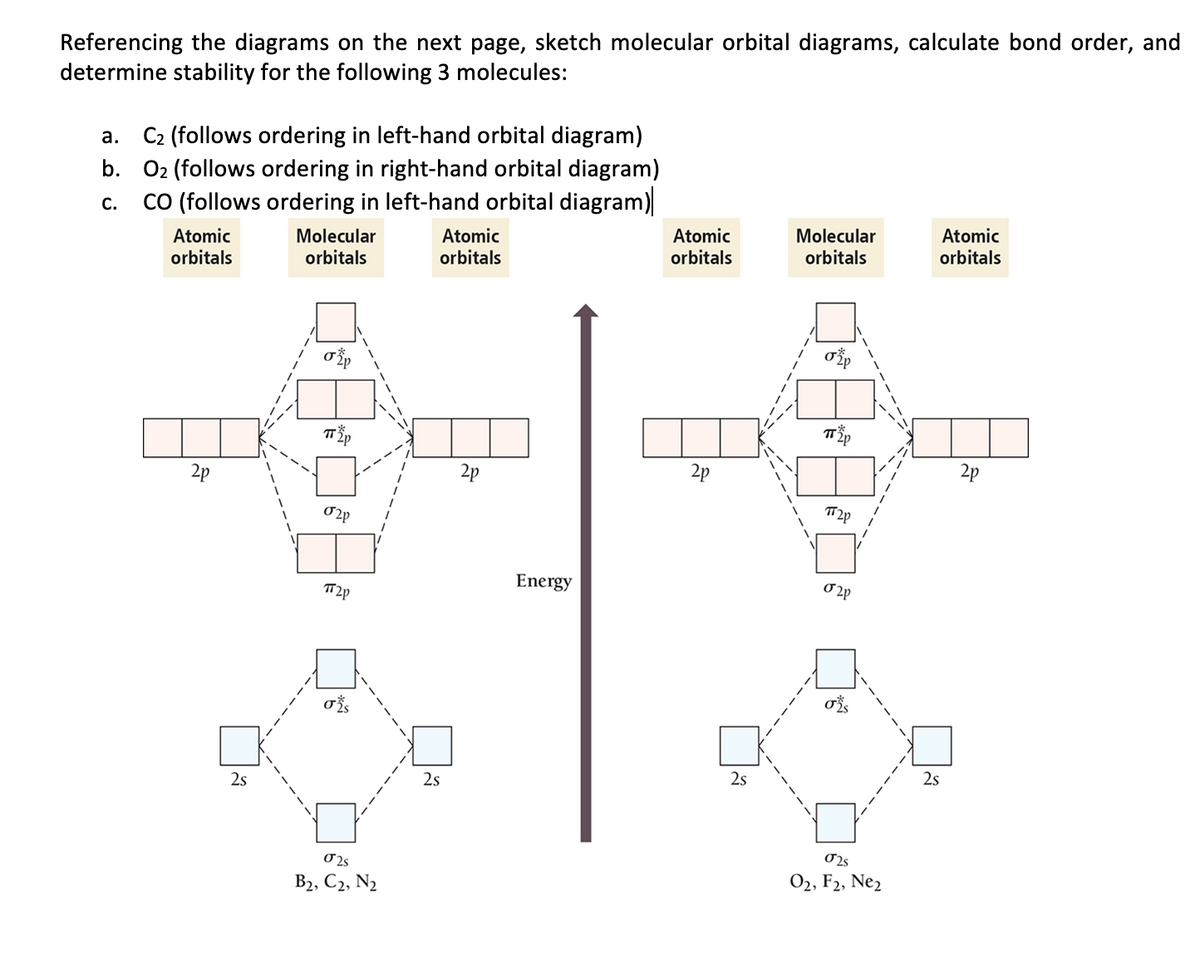
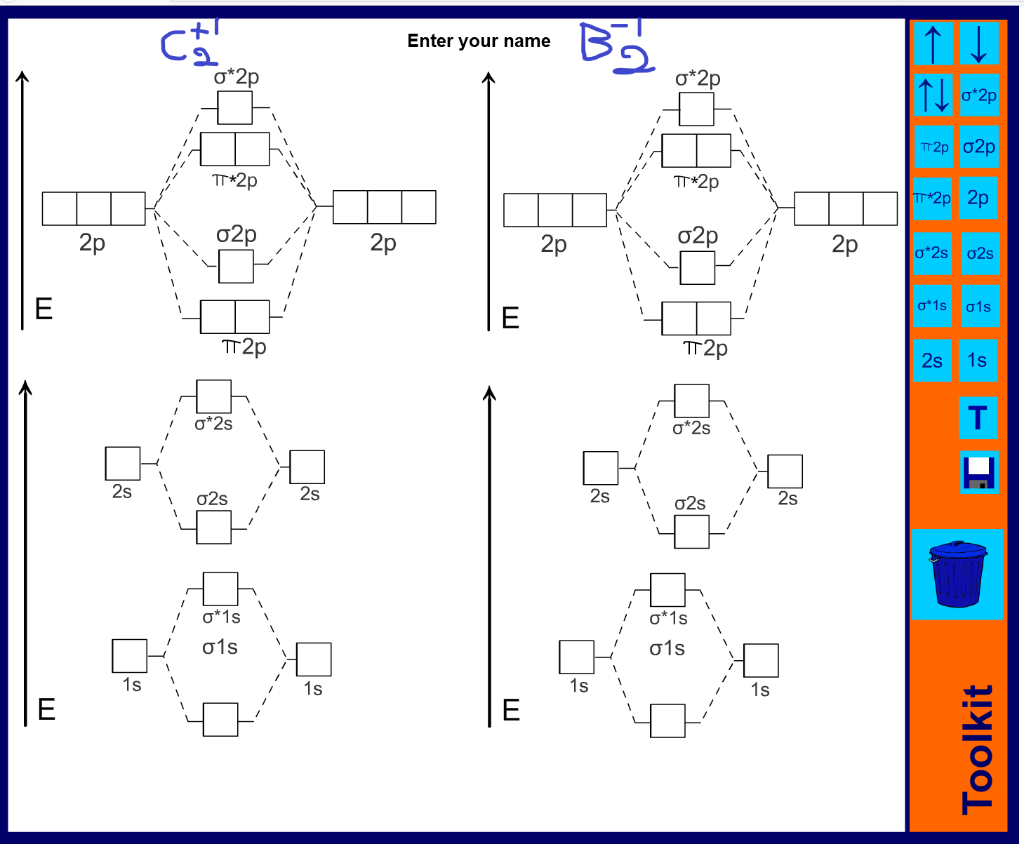


0 Response to "38 c2- molecular orbital diagram"
Post a Comment