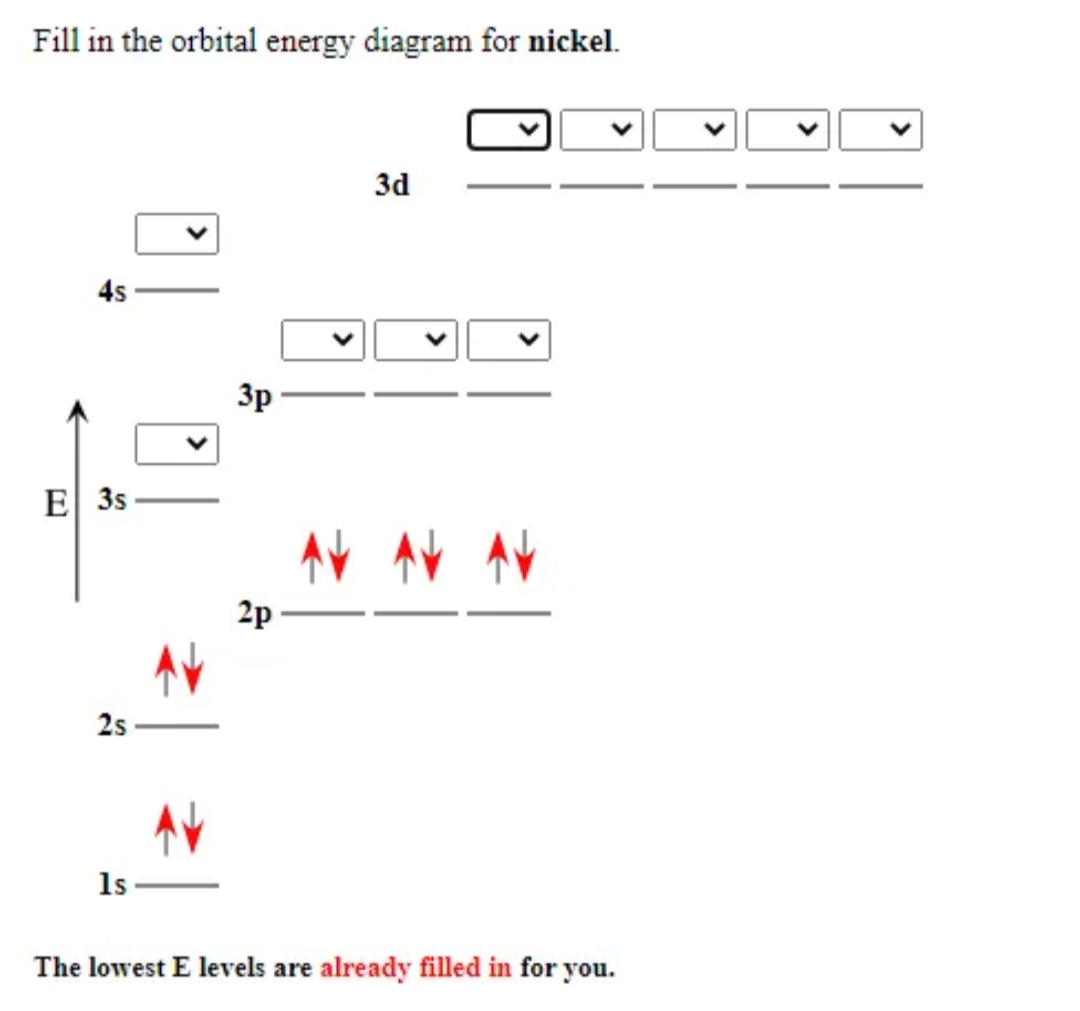
38 orbital diagram for nickel
Solved Construct the orbital diagram for nickel 1 11 1 4p ... Construct the orbital diagram for nickel 1 11 1 4p 1 11 14 1 3d 1 4s Answer Bank 1 1 1 3p 1 1 1L 3s 1 1 1L 2p 1 2s 1s Energy Construct the orbital diagram of the F ion Зр Answer Bank 3s 1 2p 2.s 1s Energy. Nickel Electron Configuration (Ni) with Orbital Diagram Nickel Electron Configuration (Ni) with Orbital Diagram. January 26, 2021 Leave a Comment. Nickel Electron Configuration: Nickel is a chemical element which has a 28 th position in the periodic table. The symbol of Nickel is "Ni" and its atomic number is 28. Nickel is a silvery-white lustrous metal which has a golden tinge.
Mir - Wikipedia Mir (Russian: Мир, IPA: ; lit. 'peace' or 'world') was a space station that operated in low Earth orbit from 1986 to 2001, operated by the Soviet Union and later by Russia. Mir was the first modular space station and was assembled in orbit from 1986 to 1996. It had a greater mass than any previous spacecraft.At the time it was the largest artificial satellite in orbit, succeeded by the ...
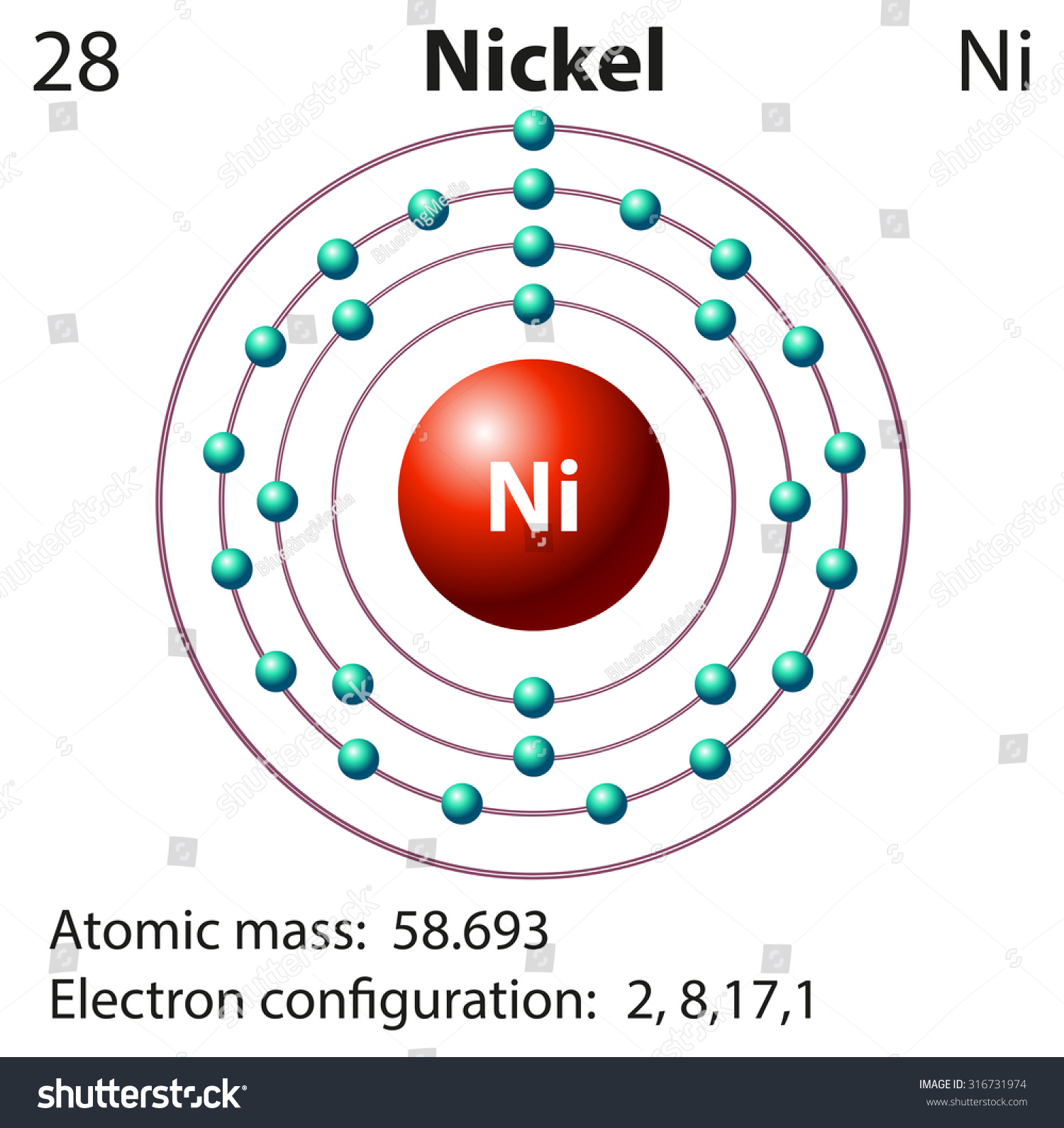
Orbital diagram for nickel
Electron Configuration Questions and Answers | Study.com Draw the orbital electron filling diagram, using the shortcut of (noble gas) to represent core electrons and up/down arrows to indicate all other electrons, for the … The Orbital Diagram for Ni Stories - bengislife.com The Orbital Diagram for Ni Stories. The methods to acquire an atomic orbital with the correct character for the bonding is known as hybridization. Frequently, the bonding atomic orbitals have a character of numerous possible kinds of orbitals. As soon as it's simple to comprehend why electrons would occupy empty orbitals before pairing up, it ... Planet - Wikipedia A planet is a large astronomical body that is neither a star nor a stellar remnant.There are competing scientific definitions of a "planet". In the dynamicist definition adopted by the International Astronomical Union (IAU), a planet is a non-stellar body that is massive enough to be rounded by its own gravity, that directly orbits a star, and that has cleared its orbital zone of …
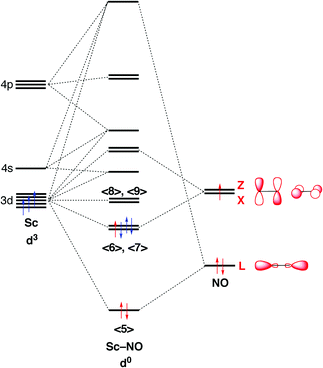
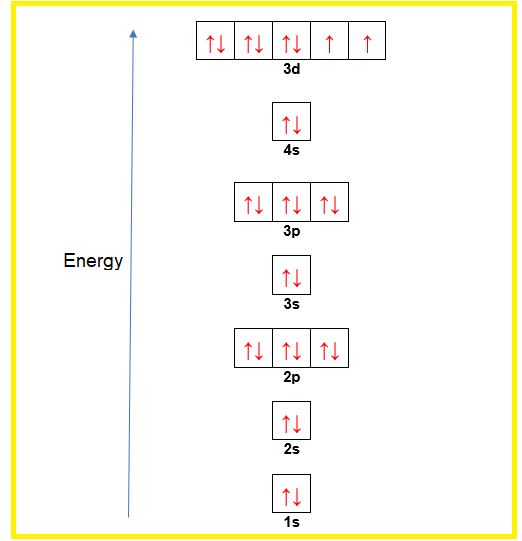
Orbital diagram for nickel. 4.3: High Spin and Low Spin Complexes - Chemistry LibreTexts 2022-01-28 · The two to go are from the 4s orbital and Nickel becomes:[Ar]4s 0 3d 8. Thus, we can see that there are eight electrons that need to be apportioned to Crystal Field Diagrams. The pairing of these electrons depends on the ligand. Since Cyanide is a strong field ligand, it will be a low spin complex. Toward 100% Spin–Orbit Torque Efficiency with High Spin ... 2022-02-25 · The sizable orbital current generation by 3d transition metals has been experimentally verified, especially in a Cr/Pt/CoFeB system. The orbital-to-spin angular momentum conversion is considered as an additional pathway to generate spin current and therefore further enhance the SOT efficiency. Here we show that Pt–V and Pt–Cr with … What is the orbital diagram of aluminum? - Quora Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. The full electron shell (includ... Construct The Orbital Diagram For Ni The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p64s2 3d8. In all of the cases, both up and down arrows are filled,with the exception of the 3d shell. Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first.
Quantum Number Questions and Answers | Study.com How many electrons with orbital quantum numbers l = 2 does the nickel atom have? View Answer If the magnitude of the nuclear, spinangular momentum of a nuclei, is 15/2 units. Construct The Orbital Diagram For Ni The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p64s2 3d8. In all of the cases, both up and down arrows are filled,with the exception of the 3d shell, where the last two are.What is the orbital diagram for nickel? | schematron.orgWhat is the orbital diagram for nickel. What is the orbital diagram for nickel? | Study.com What is the orbital diagram for nickel? Atomic Orbital Diagrams: Atomic orbital diagrams are also known as electron-in-a-box diagrams. These are simplified diagrams of how electrons are arranged ... PDF Hybrid Molecular Orbitals - University of Illinois Urbana ... molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a
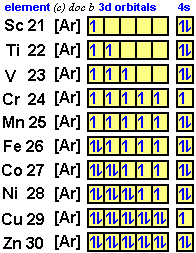
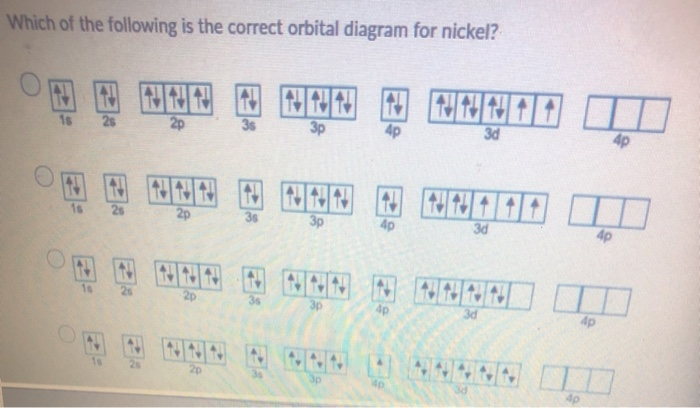
Arrangements of electrons in the orbitals of an atom is ... The orbital diagram for hydrogen can be represented in the following way. This notation uses a box to represent the orbital, the label for the orbital and an arrow to represent the electron. ... From iron through nickel ([Ar]4s 2 3d 6, [Ar]4s 2 3d 7, [Ar]4s 2 3d 8) the electrons spin-pair in the 3d sublevel. At copper another reversal occurs. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: QUESTION 24 Draw the orbital diagram for ... - Physical ... Question: QUESTION 24 Draw the orbital diagram for nickel (Ni). How many unpaired electrons does Ni have and is Ni paramagnetic or diamagn Solved Construct the orbital diagram for nickel. Answer ... Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 90% (20 ratings) Transcribed image text: Construct the orbital diagram for nickel. Answer Bank 111 Energy.
What is the orbital diagram for nickel? - Quora Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...
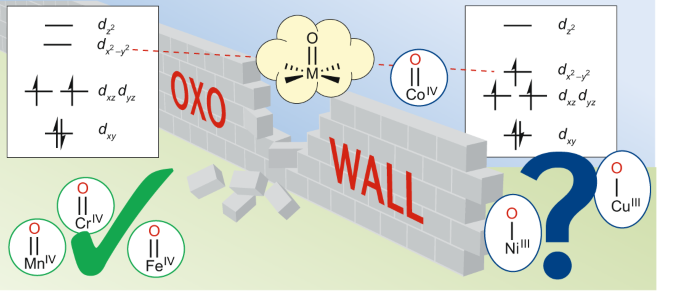
Design and tailoring of carbon-Al2O3 double coated nickel ... (b) Schematic diagram of the band structure in Ni-based cation-disordered (DRS) oxides changing with lattice parameter a. The light green arrows represent the extent of overlap between Ni e g * and unhybridized O 2p bands. Smaller a originated from small Al 3+ substitution elevates the Ni band, which signifies the smaller overlap. (c) Schematic ...
Nickel(Ni) electron configuration and orbital diagram To write the orbital diagram of nickel(Ni), you have to do the electron configuration of nickel. Which has been discussed in detail above. Nickel(Ni) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter ...
Electron configuration for Nickel (element 28). Orbital ... Ni (Nickel) is an element with position number 28 in the periodic table. Located in the IV period. Melting point: 1453 ℃. Density: 8.91 g/cm 3 . Electronic configuration of the Nickel atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8. Electronic configuration of the Nickel atom in ascending order of the levels:
What is the orbital diagram for nickel? - Answers The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ...
Orbital Diagram For Arsenic - schematron.org Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!
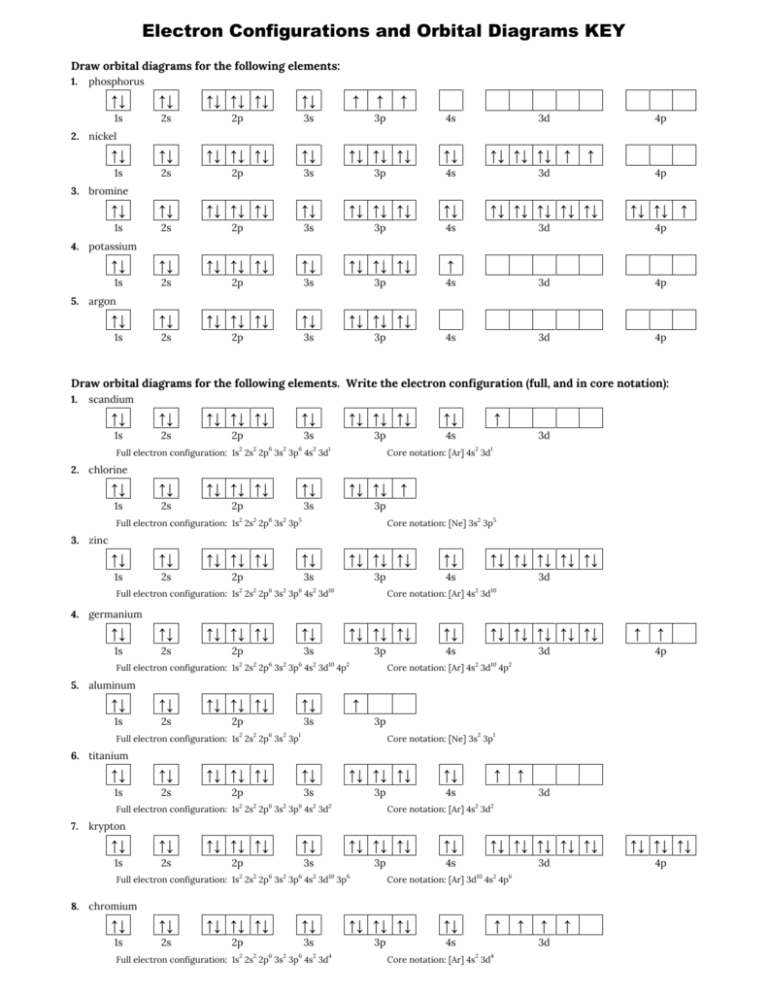
Electron Config & Orbital Filling Answer Key Write the full electron configuration, noble gas electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitro en 3s B 2. Chlorine Is 3. Sodium a øan a as ap 4. Neon Is 23 5. Nickel 6 Vanadium Is 7 Cop er
What is the electron configuration for nickel, whose ... Explanation: Nickel is in the 4th energy level, d block, 7th column, this means that the electron configuration will end 3d8 with the d orbital being one level lower than the energy level it is on. N i = 1s22s22p63s23p64s23d8. N i = [Ar]4s23d8. YouTube.
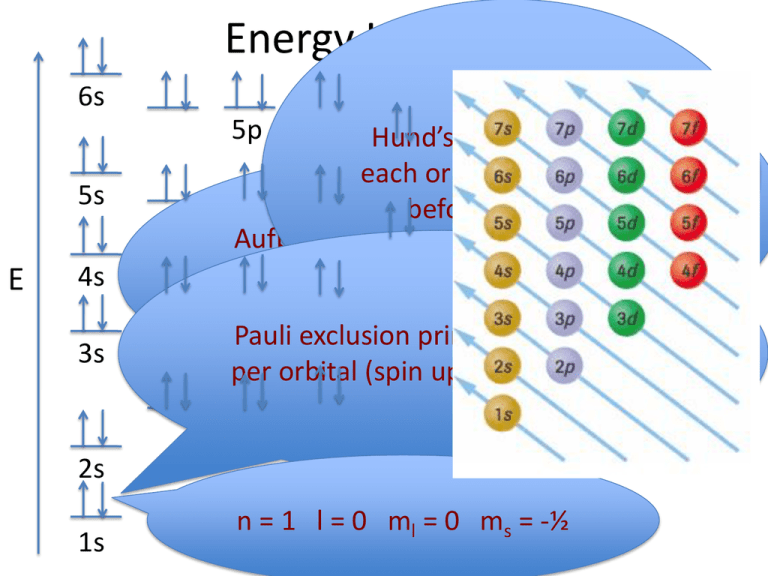
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
Manganese(Mn) electron configuration and orbital diagram Atomic Orbital Diagram for Manganese (Mn) Manganese(Mn) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of manganese is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 2. The valency of the element is determined by electron configuration ...
How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
What is the orbital diagram for Helium? - AskingLot.com What is the orbital diagram for Helium? Helium only has 2 electrons and therefore it has a configuration of 1s2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas.
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Electron Configuration for Ni, Ni2+, and Ni3+ (Nickel and ... To write the configuration for the Nickel ions, first we need to write the electron configuration for just Nickel (Ni). We first need to find the number of ...
How many unpaired electrons should there be in an orbital ... N i2+ looses electrons from the 4s orbital and has 8 electrons in the 3d orbital resulting in 2 unpaired electrons. Atomic number of N i is 28. There are two unpaired electrons. Hence,option B is correct. What happens when nickel becomes ni ^ + 2? When Nickel becomes Ni^+2 Nickel has lost 2 electrons leaving the atom with only 8 valance electrons.
Molecular engineering of dispersed nickel phthalocyanines ... 2020-08-10 · The Gibbs free energy diagram of the CO 2 RR on NiPc from DFT calculations showed that the adsorption of CO 2 on the active nickel centre was accompanied by a proton-coupled electron transfer step ...
PDF Electron Configuration and Orbital Filling Diagram WS Key Electron Configuration & Orbital Filling Diagram WS Usinq the Lonq Method, qive the Electron Configuration: Magnesium (Mg): Potassium (K): Lithium (Li): Nickel (Ni): Identi the followin Elements: Is2 2s2 2p2: 1s2 2s2 2p6. [Arl 4s2 3d10 4p5 Órbital Fillinq Diaqrams Is 2s 2P is the element 3p 12. c 13. Ni (short cut) Identifv the followinq elements:
Planet - Wikipedia A planet is a large astronomical body that is neither a star nor a stellar remnant.There are competing scientific definitions of a "planet". In the dynamicist definition adopted by the International Astronomical Union (IAU), a planet is a non-stellar body that is massive enough to be rounded by its own gravity, that directly orbits a star, and that has cleared its orbital zone of …
The Orbital Diagram for Ni Stories - bengislife.com The Orbital Diagram for Ni Stories. The methods to acquire an atomic orbital with the correct character for the bonding is known as hybridization. Frequently, the bonding atomic orbitals have a character of numerous possible kinds of orbitals. As soon as it's simple to comprehend why electrons would occupy empty orbitals before pairing up, it ...
Electron Configuration Questions and Answers | Study.com Draw the orbital electron filling diagram, using the shortcut of (noble gas) to represent core electrons and up/down arrows to indicate all other electrons, for the …













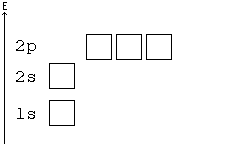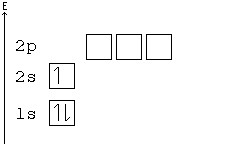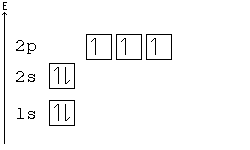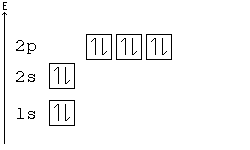


![ii. [CoCl,]2® is tetrahedral complex. Draw its+ box orbital ...](https://hi-static.z-dn.net/files/d55/d2a5d007d0ea85944329473e340d4d92.png)



![CHEMSOLVE.NET: [Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 ...](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiCxKQPooF4tNqoZeVyqocZ8nc_05A6aVkvR6kFA6BDaMbTzvU_qrwEjPIYprdXD9lQcsJcAjlPo_J0JHH3T0RnR3sIK2wkRvduvMS7UznhTOGkGXhqDmlOKXEoeE9r3FRmIIuNvTxAoLk/s640/koil+-+Copy.jpg)


0 Response to "38 orbital diagram for nickel"
Post a Comment