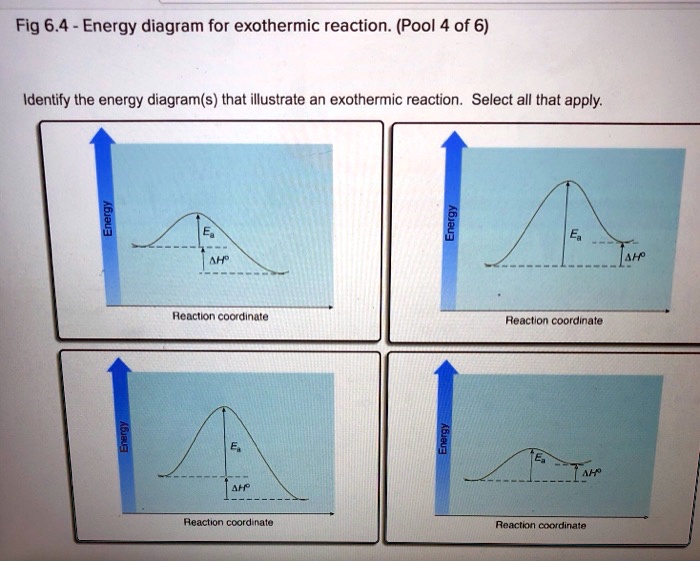
40 reaction coordinate diagram exothermic
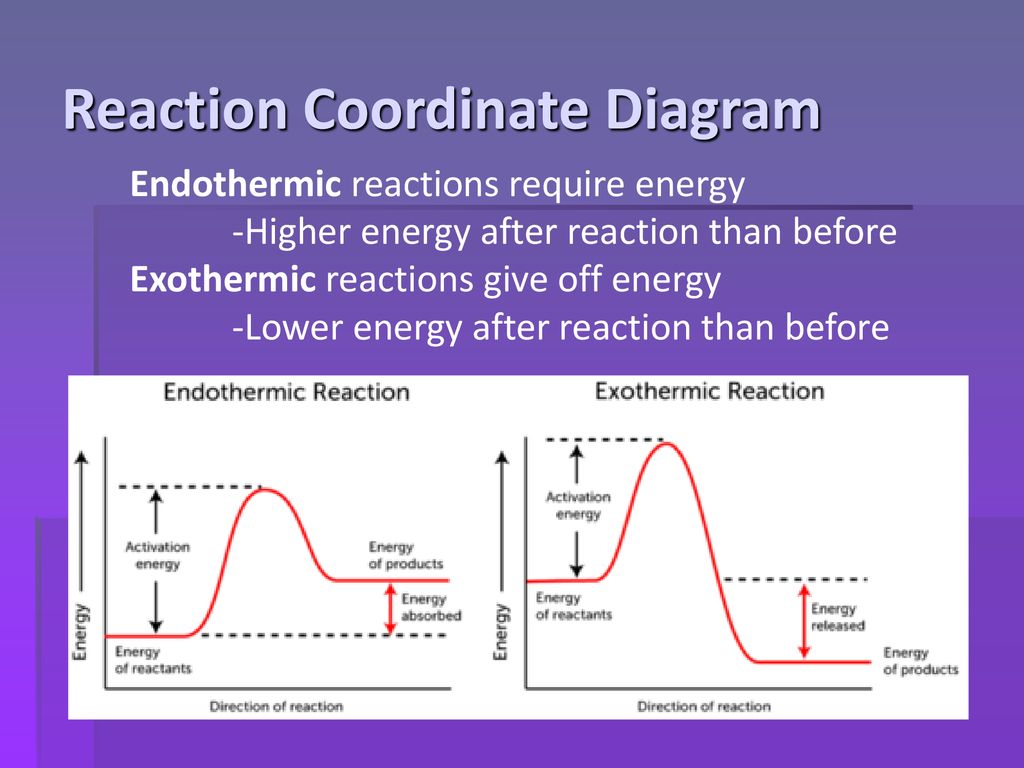
Reaction Coordinate Diagram Endothermic Vs Exothermic A reaction will be exothermic if the energy of the products is less than the energy of the Below is a reaction coordinate diagram for an endothermic reaction. A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction. Endothermic Reaction Coordinate Diagram - Wiring Diagrams The diagram below is called a reaction coordinate diagram. B is at a lower total energy than A. This is an exothermic reaction (heat is given off).reaction pathway. They do not predict the rate of reaction, which depends on pathway.! • The reaction rate (the number of effective collisions in a period of time) relates to many factors. 1.
Potential Energy Diagrams - Chemistry - Catalyst, Endothermic & Exothermic ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
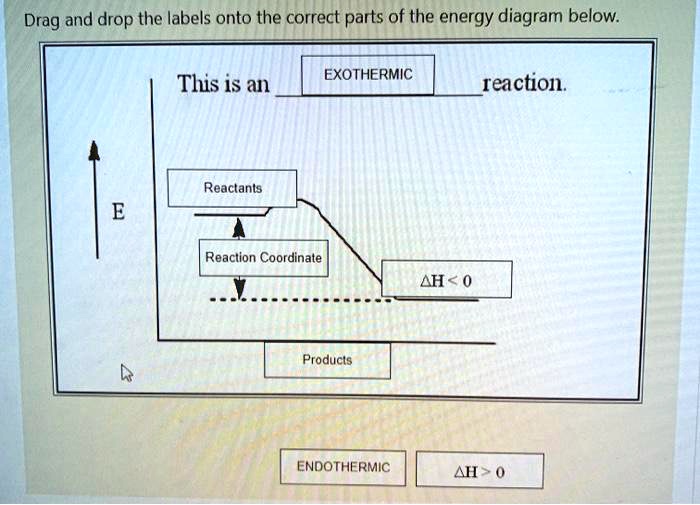
Reaction coordinate diagram exothermic
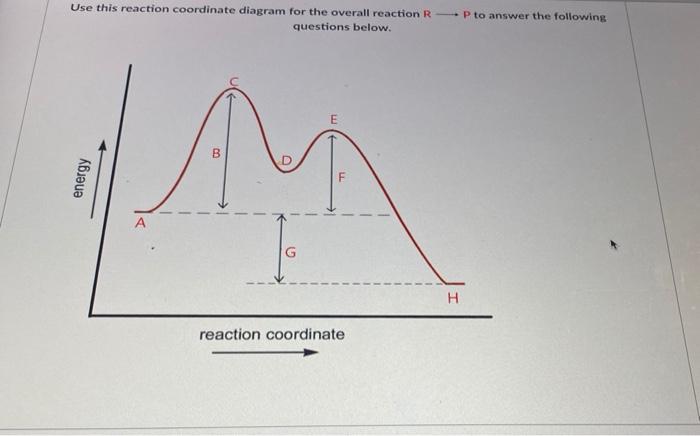
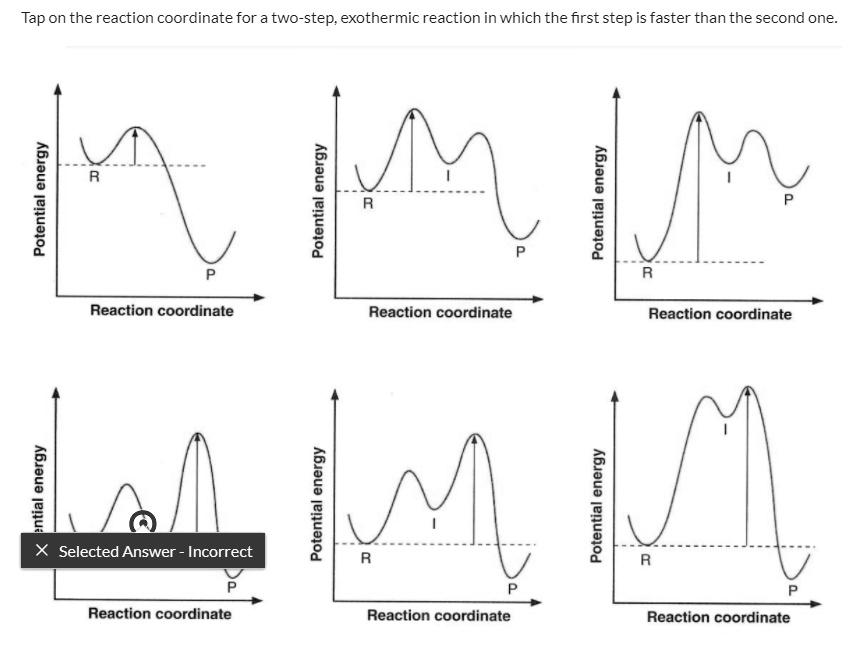
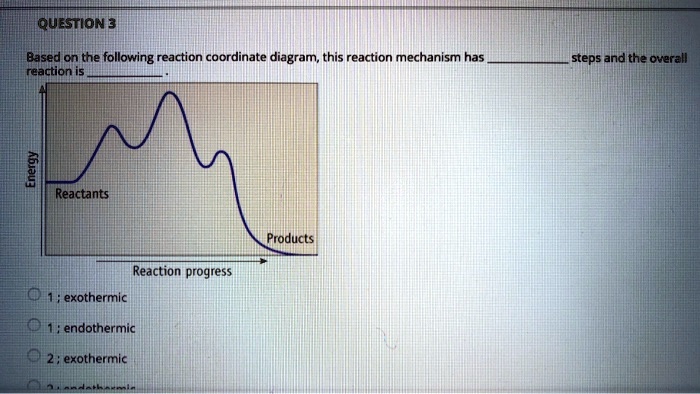
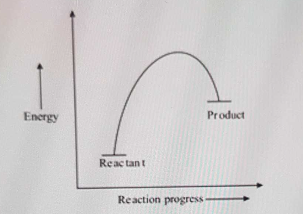
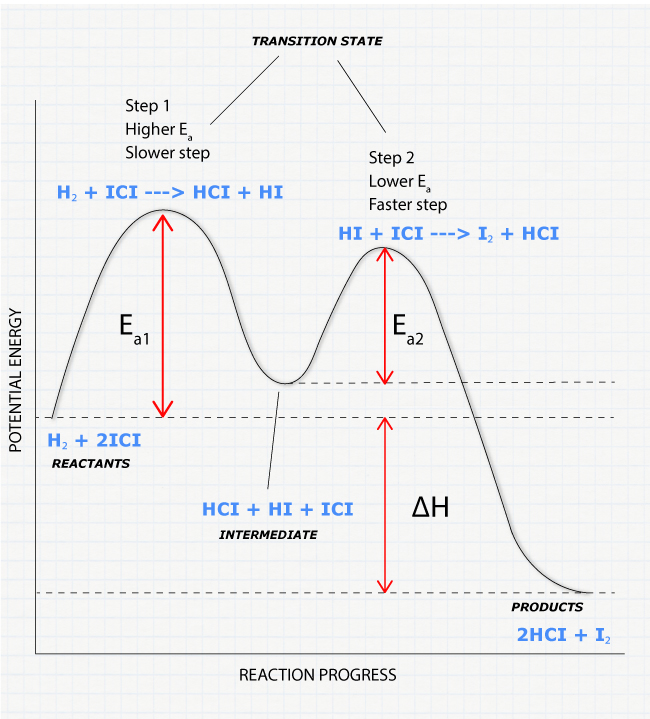
Exam 2 (part 2 - reaction coordinate diagram) Flashcards | Quizlet Reaction Coordinate Diagram. a graph that shows how the energy of a reaction changes as the reaction progresses. The most important parts of the energy diagram are the energy levels for the starting materials, transition state (s) and the final product (s). -Products will be more stable than reactants. Rate Determining Step for Reaction Coordinate Diagram | Student Doctor Network Let's say you're looking at a reaction coordinate diagram for an exothermic reaction. The reaction has two transition states, the first transition state having a lower hill than the second transition state. Also, the first transition state has the larger activation energy compared to the second transition state. Is Melting Exothermic or Endothermic? - Techiescientist The given diagram is called a reaction coordinate diagram. In this, the energy of species is plotted as a function of reaction progress. It represents an exothermic reaction. For example, respiration is an exothermic process. Respiration is defined as the oxidation of food to release energy. Since energy is released, it is an exothermic process.
Reaction coordinate diagram exothermic. Reaction Coordinate Diagram Endothermic - Wiring Diagrams The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions.A reaction will be exothermic if the energy of the products is less than the energy of the reactants. A reaction is endothermic when the energy of the products is greater than the energy of the reactants. Potential energy diagram with/without catalyst in a hypothetical ... Potential energy diagram with/without catalyst in a hypothetical exothermic chemical reaction coordinate of Boltzmann distribution. The presence of the catalyst opens a different reaction pathway... How To Draw A Reaction Coordinate Diagram | Know Your Care This is an exothermic reaction (heat is given off) and should be. It Shows How The Energy Of The System Changes During A Chemical Reaction. A man is pushing a 10kg box on a rough floor, with a coefficient of friction of µ = 0.6, by applying a 20n force. The reaction pathway on this plot is t. Draw a reaction coordinate diagram (energy vs. Quick Answer: How To Draw A Reaction Coordinate Diagram? How do you graph a reaction? 1 Response Draw and label a pair of axes, with the vertical axis labeled "Potential Energy" and the horizontal axis labeled "Reaction Coordinate." Draw and label two short horizontal lines to mark the energies of the reactants and products. Draw and label the energy level diagram.
Exothermic Energy Diagram: Activation Energy, Transition States ... - YouTube In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ... › science › articleUncovering electrocatalytic conversion mechanisms from Li2S2 ... An exothermic Li 2 S 2 RR in U = 0 V can be endothermic at E o, thus is not spontaneous anymore. U L in all *Li 3 S 2 and *LiS associative pathways is listed in Table 1. For *LiS associative reaction pathway, the lowest η theo is provided by [email protected], with a value of 0.38 V vs. Li/Li +. lambdageeks.com › endothermic-reaction-examples12+ Endothermic Reaction Examples: Detailed Explanations endothermic reaction examples: In Chemistry, endothermic reaction is defined as one type of reaction in which any system absorbs energy in form of heat, light from surroundings. Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. This means that there was more energy in reactants than in the products.
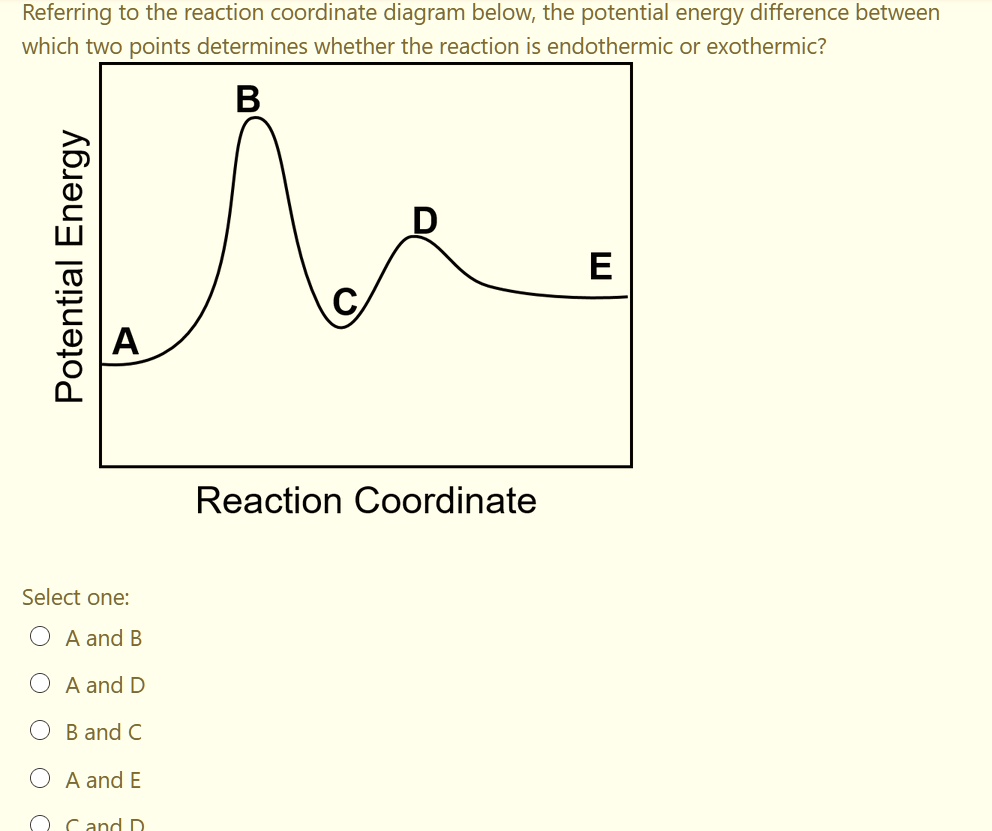
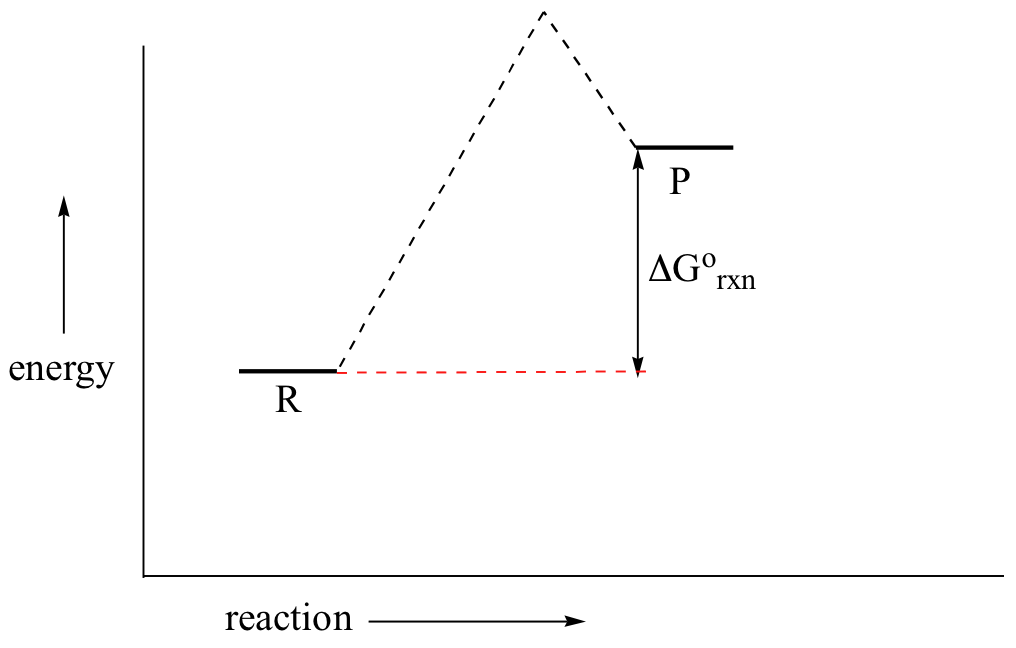
pediaa.com › difference-between-reactants-and-productsDifference Between Reactants and Products | Definition ... Jul 20, 2017 · Figure 01: The reaction-coordinate diagram for an exothermic chemical reaction The above image shows a reaction-coordinate diagram for a particular chemical reaction. In this reaction, the reactants have a high potential energy than the products. Question 24 Draw a reaction coordinate dia... - Organic Chemistry Question 24 Draw a reaction coordinate diagram for the reaction above (question 23). Assume it is an exothermic reaction. Label the products, reactants, intermediates, transition state, the activation energy and the overall energy of the transformation Endothermic Reaction Coordinate Diagram - schematron.org The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other.Start studying CHEMISTRY 2. › topics › engineeringArrhenius Equation - an overview | ScienceDirect Topics Reaction coordinate diagram for an exothermic reaction. 3.7.2.1 First principle–based approaches3.7.2.1.1 Transition State Theory. The power of DFT is well established.
Is Melting Exothermic or Endothermic? - Techiescientist The given diagram is called a reaction coordinate diagram. In this, the energy of species is plotted as a function of reaction progress. It represents an exothermic reaction. For example, respiration is an exothermic process. Respiration is defined as the oxidation of food to release energy. Since energy is released, it is an exothermic process.
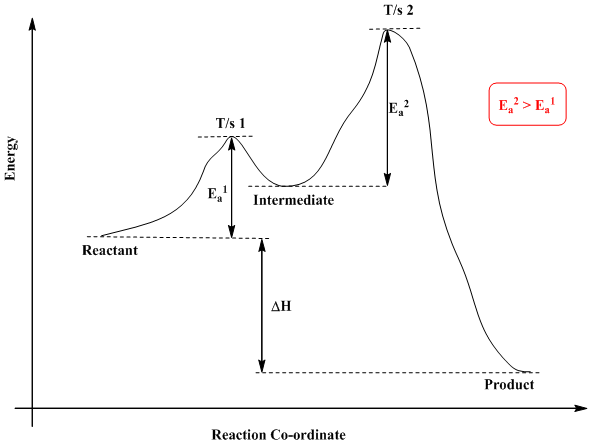
Rate Determining Step for Reaction Coordinate Diagram | Student Doctor Network Let's say you're looking at a reaction coordinate diagram for an exothermic reaction. The reaction has two transition states, the first transition state having a lower hill than the second transition state. Also, the first transition state has the larger activation energy compared to the second transition state.
Exam 2 (part 2 - reaction coordinate diagram) Flashcards | Quizlet Reaction Coordinate Diagram. a graph that shows how the energy of a reaction changes as the reaction progresses. The most important parts of the energy diagram are the energy levels for the starting materials, transition state (s) and the final product (s). -Products will be more stable than reactants.




















0 Response to "40 reaction coordinate diagram exothermic"
Post a Comment