41 nitrogen atomic orbital diagram
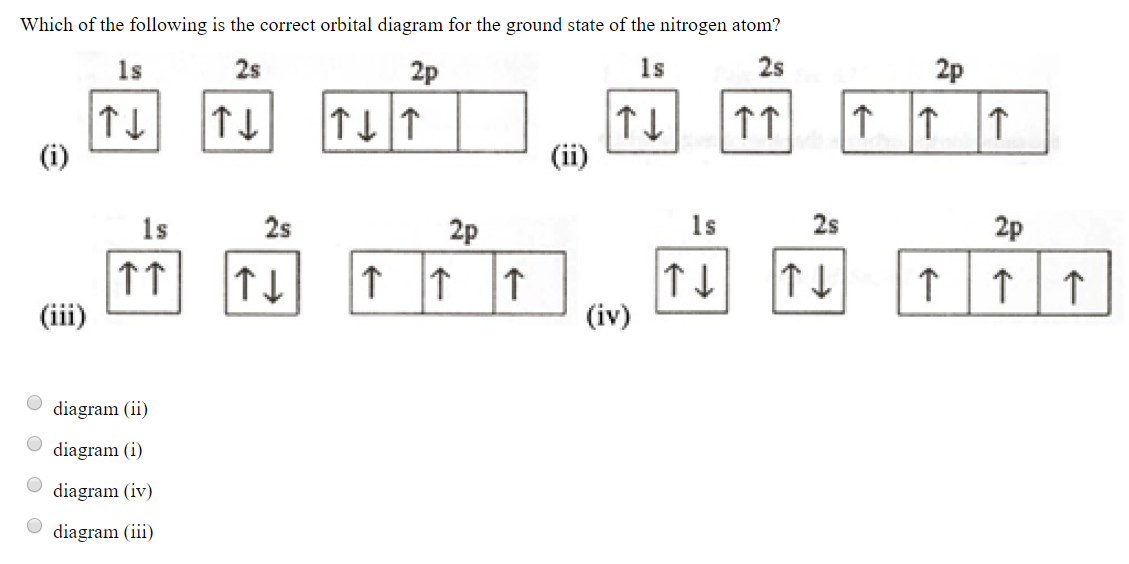
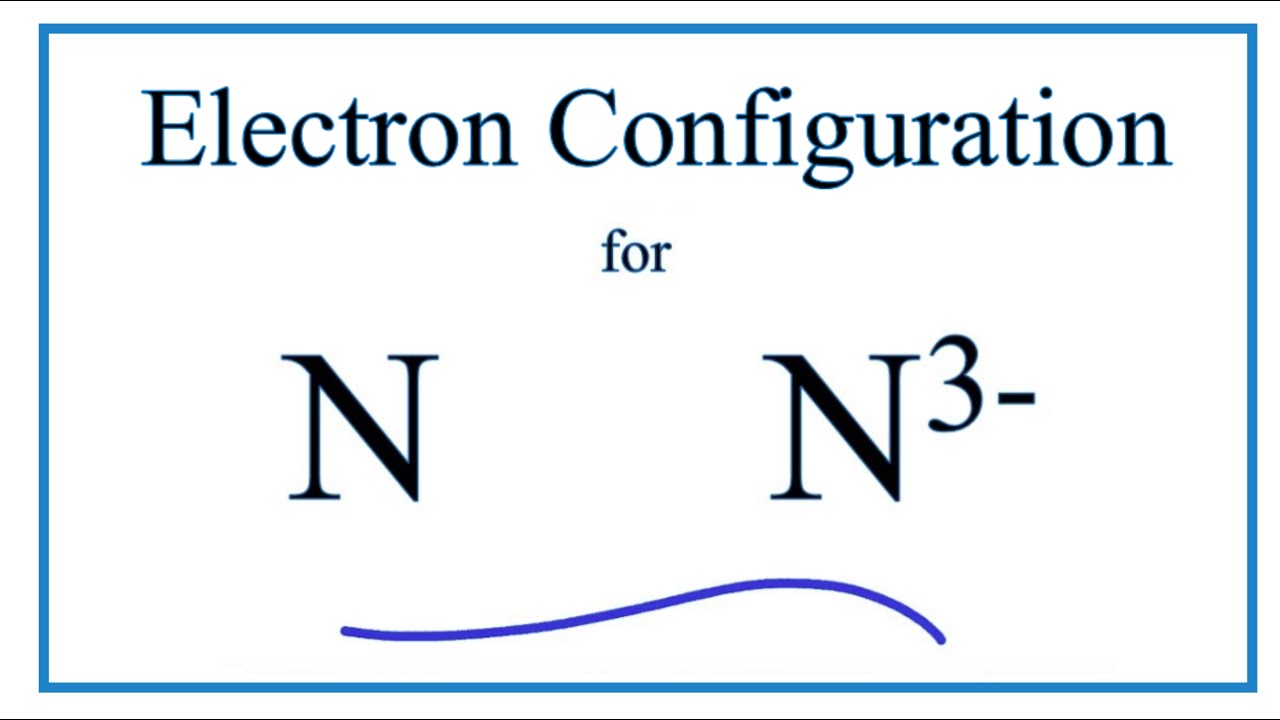
⚗️Which orbital diagram represents nitrogen (atomic number ... The choice A accurately specifies and illustrates the orbital diagram of a Nitrogen atom with 7 electrons. Based on the number of electrons in a Nitrogen atom, there are two energy levels, the s and p sub-levels: Nitrogen = 2, 5 The first energy level, S will take up two electrons with opposite spin. Nitrogen, atomic structure - Stock Image - C018/3688 ... Nitrogen (N). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of nitrogen-14 (atomic number: 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings).
Orbital Filling Diagram For Nitrogen - schematron.org If that single electron were a spin-up (ms = +1/2), the orbital diagram for The figure below illustrating orbital diagrams for nitrogen is similar to the. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p .

Nitrogen atomic orbital diagram
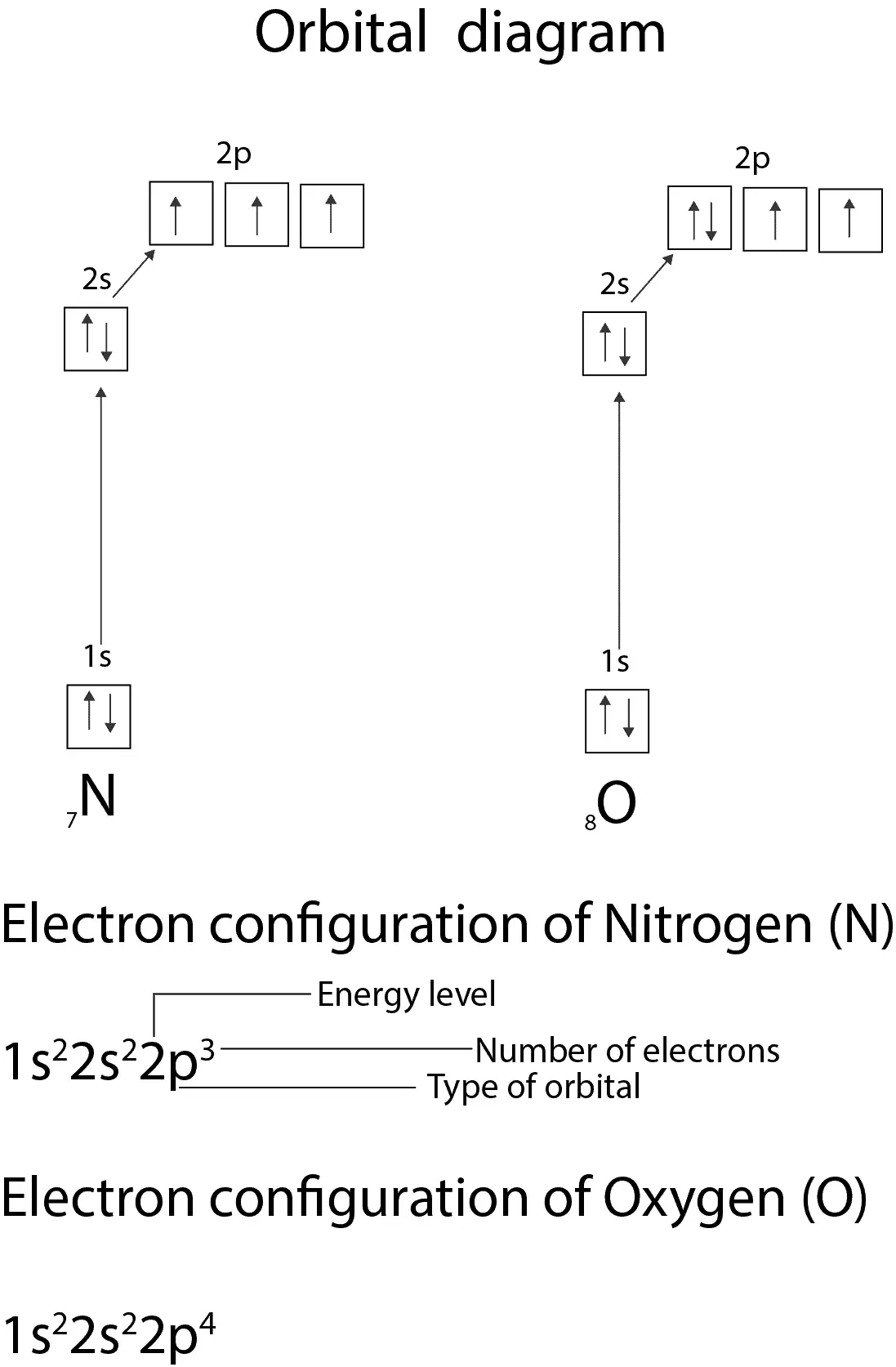
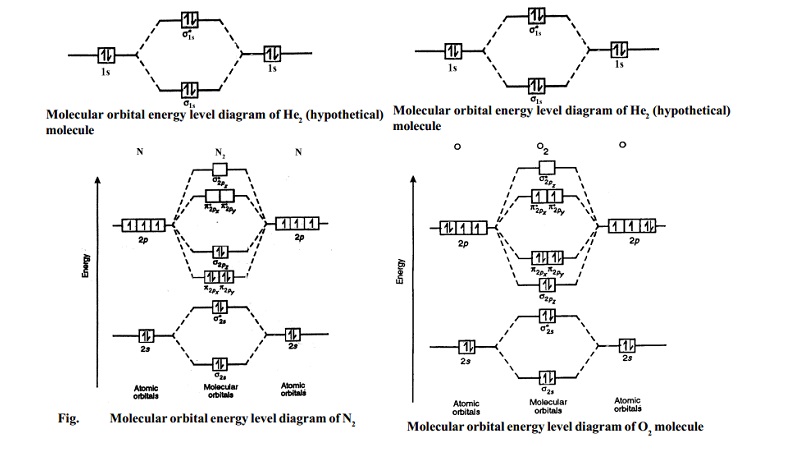
Molecular Nitrogen and Related Diatomic Molecules Here is the full molecular orbital diagram for N2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively. Oxygen(O) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of oxygen atom through orbital. Atomic energy levels are subdivided into sub-energy levels. Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z.
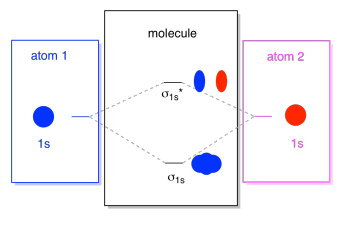
Nitrogen atomic orbital diagram. Atomic and Molecular Orbitals - Michigan State University In general, this mixing of n atomic orbitals always generates n molecular orbitals. The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. What Is The Hybridization Of Atomic Orbitals Of Nitrogen ... TheHybridization Of Atomic Orbitals Of Nitrogen In NO+2. The total electrons in the valence shell are 16. 16 / 8= 2. Quotient = 2 and Remainder =0. 2+0/2=2. Therefore The hybridization of atomic orbitals of nitrogen in NO+2 is Sp. TheHybridization Of Atomic Orbitals Of Nitrogen In NO-3. The total electrons in the valence shell are 32. 32 / 8 = 4. Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Apart from that one more thing is unique about the element, i.e, nitrogen can have either one of 3 or 5 valence electrons. What is the atomic orbital diagram for nitrogen? | Study.com Atomic Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals for a particular atomic species.
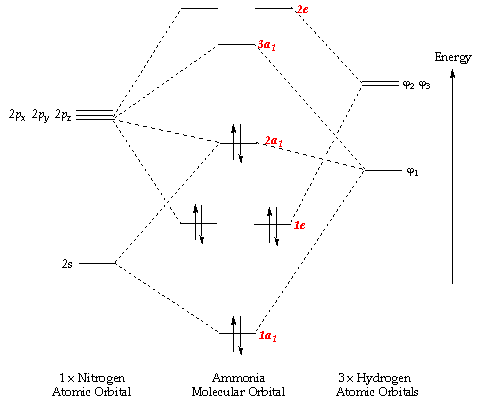
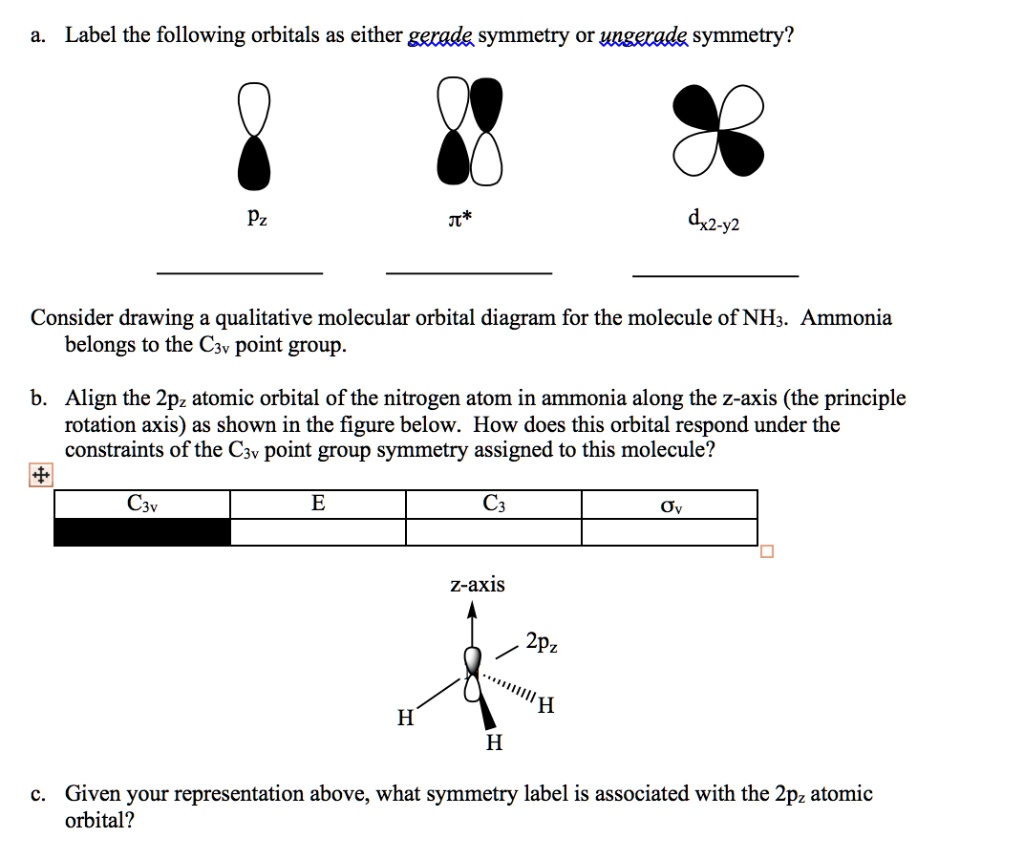
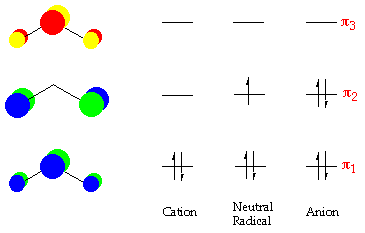
Nitrogen - Wikipedia Molecular orbital diagram of dinitrogen molecule, N 2. There are five bonding orbitals and two antibonding orbitals (marked with an asterisk; orbitals involving the inner 1s electrons not shown), giving a total bond order of three. Atomic nitrogen, also known as active nitrogen, is highly reactive, being a triradical with three unpaired electrons. Sulfur Orbital diagram, Electron configuration, and Valence ... Orbital diagram:-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons. Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals. It shows the electrons in ... Orbital Filling Diagram For Nitrogen - wiringall.com Figure 1. The 2p .Show transcribed image text Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. 6.2.4: NH3 - Chemistry LibreTexts Construct SALCs and the molecular orbital diagram for NH 3. Preliminary Steps Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principle axis. Step 2. Identify and count the pendant atoms' valence orbitals. Generate SALCs Step 3. Generate the Γ 's Step 4.
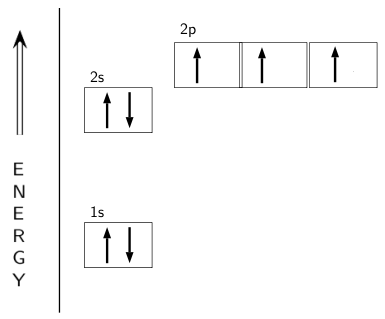
Electron Configuration for Nitrogen (N) - UMD In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s 2 2s 2 2p 3. CN- lewis structure, molecular orbital diagram, and, bond order Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3. 8 - Drawing Molecular Orbital Diagrams — Flux Science Nov 12, 2021 · Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. Although more complex, these diagrams reveal a more realistic case for bonding, allowing electrons to travel about a molecule, rather than in between one. Fluorine(F) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagrams. Electron configuration of fluorine through orbital. Atomic energy levels are subdivided into sub-energy levels.
Draw the molecular orbital diagram of N2N2 + N2 Write ... Basic structure of molecular orbital diagram for nitrogen is: Electrons of nitrogen are to be filled in this diagram. Left side represents the configuration of one atom of nitrogen molecule and the right side represents the second atom of nitrogen molecule. Atomic number of nitrogen is seven. Therefore in N 2 there are a total fourteen electrons.
Electron Configuration Orbital Diagram Nitrogen - YouTube To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.
Molecular orbitals in Nitrogen - ChemTube3D There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two perpendicular pi bonds. Three filled bonding orbitals… … and three empty antibonding orbitals.
Nitrogen, atomic structure - Stock Image - C013/1506 ... Nitrogen (N). Diagram showing the nuclear composition and electron configuration of an atom of nitrogen-14 (atomic number: 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
Nitrogen Bohr Model - How to draw Bohr diagram for ... Summary. The Bohr model of Nitrogen is drawn with only two electron shells, the first shell contains 2 electrons and the second shell contains 5 electrons. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its Bohr diagram is also 7. The number of neutrons for the Bohr diagram of Nitrogen ...
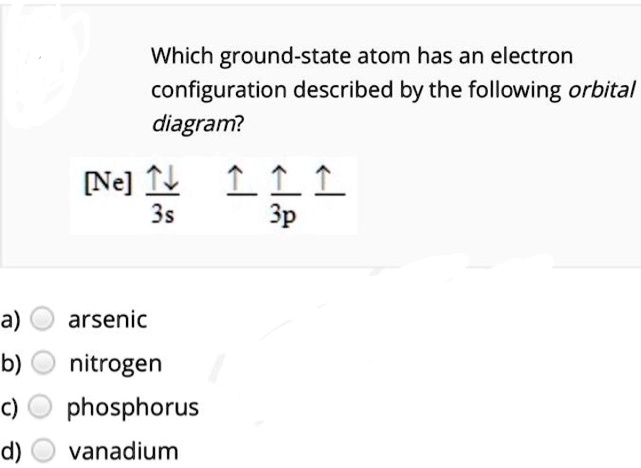
Solved Create the atomic orbital diagram for nitrogen ... Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so you need to show the 2p subshell and everything below it. Next, click the orbitals to add electrons (represented as arrows). For boron, you would need to show a total of five electrons.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
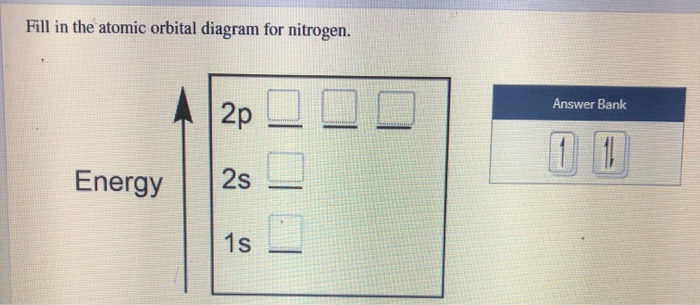
Solved Fill in the atomic orbital diagram for nitrogen ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (18 ratings) Transcribed image text: Fill in the atomic orbital diagram for nitrogen. Answer Bank Energy Construct the orbital diagram for nickel. 1000 Answer Bank Energy 2 _ _ _.
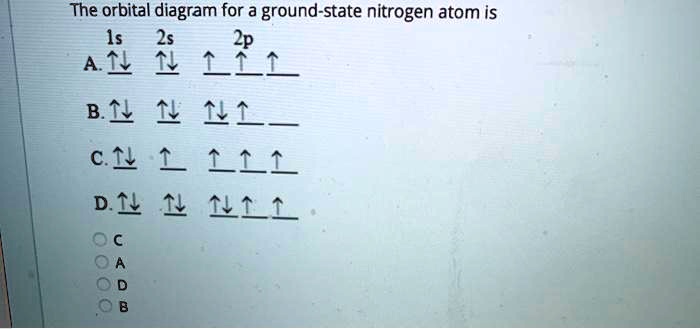
Nitrogen Orbital diagram, Electron configuration, and ... The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and the rest three electrons in the 2p orbital. The orbital diagram for a ground-state electron configuration of a nitrogen atom is as follows-
Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Nitrogen | N2 - PubChem Nitrogen sinks in some knee structure or nearby structure could be physical, chemical, or physiological. The authors conclude that these unexpected results of a very marked delay in knee gas excretion 30 minutes into the pulmonary washout period suggests that a gas exchange model consistent with these data is needed to avoid decompression sickness.
Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen Electron configuration of nitrogen in the excited state Atoms can jump from one orbital to another in an excited state. This is called quantum jump. The ground-state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z.
Oxygen(O) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of oxygen atom through orbital. Atomic energy levels are subdivided into sub-energy levels.
Molecular Nitrogen and Related Diatomic Molecules Here is the full molecular orbital diagram for N2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively.
















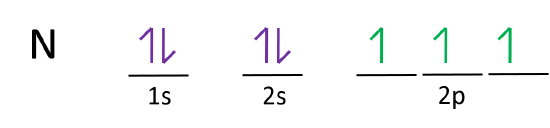


0 Response to "41 nitrogen atomic orbital diagram"
Post a Comment