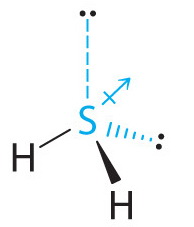
38 lewis dot diagram for h2s
SiS2 Lewis Structure, Molecular Geometry ... - Techiescientist 2 days ago · Step 5: Now assimilate all the aforementioned steps and draw the Lewis diagram of Silicon disulfide: Molecular Geometry of Silicon disulfide (SiS2) Molecular geometry is a dimensional drawing of the atoms that are forming a molecule. Through molecular geometry bond length, bond type, bond angle and other geometrical parameters can be studied ... Lewis structure calculator | Lewis structure generator Lewis structures can be used to represent valence shell electrons in a chemical bond. The Lewis structure, proposed by Gilbert Newton Lewis, who introduced it for the first time in 1916, is a graphic representation of the sharing of electrons that occurs in chemical bonds between atoms of the same or different species.
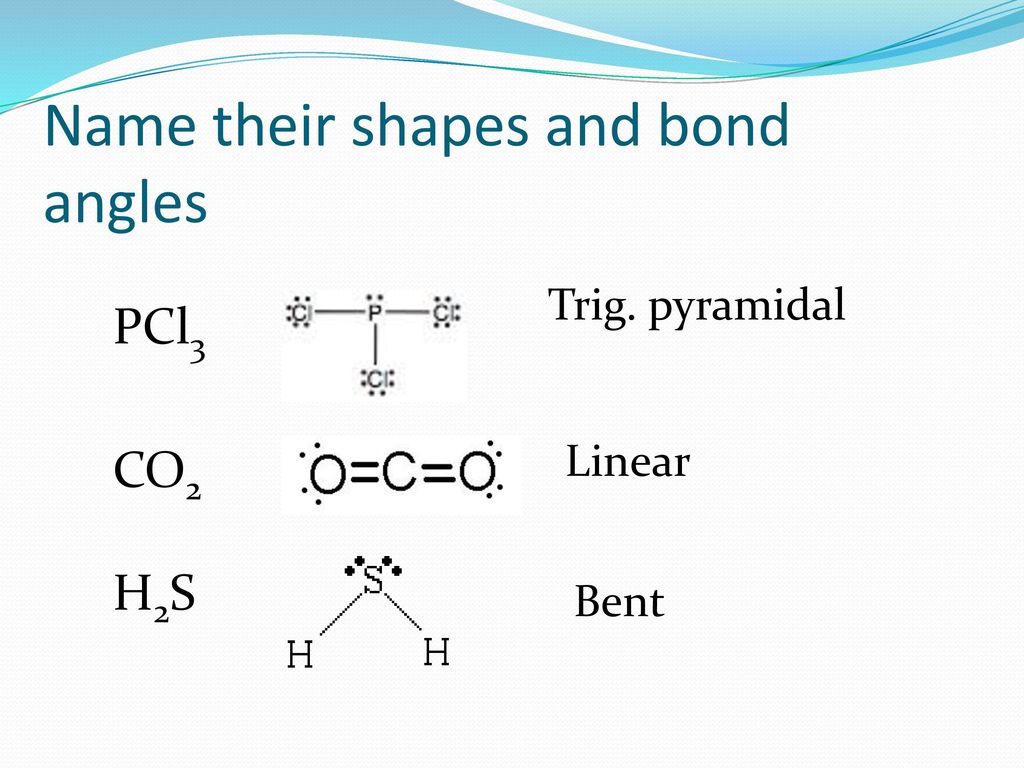
H2S Molecular geometry, SH2 Lewis structure, Bond angle, Shape Follow these steps to draw the lewis dot structure for H2S Step 1: In the first step, determine the total valence electron present in H2S. As we know hydrogen only has one valence electron in its last shell and Sulfur belongs to the 16th group in the periodic table so it contains 6 electrons in its last shell.
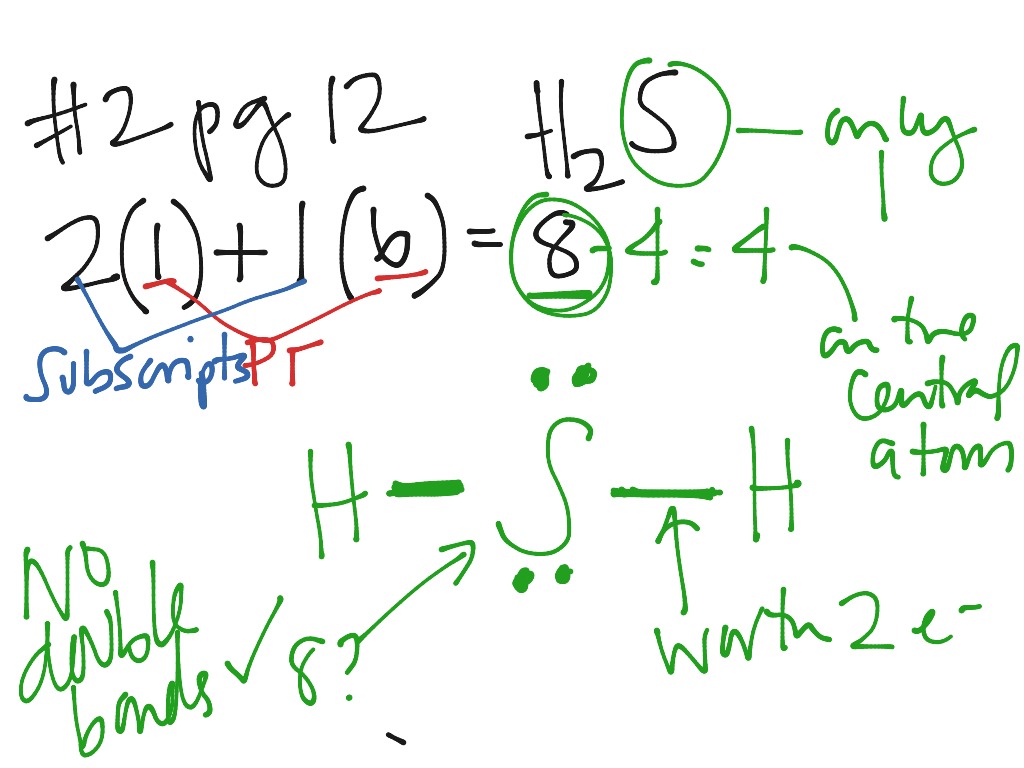
Lewis dot diagram for h2s
What Is The Lewis Dot Structure For H2S? - XpCourse There is an EASY way, and a FORMAL way to draw the Lewis structure of H 2 S, hydrogen sulfide: Formal Way. In the formal way we find how many electrons we have (step 1), how many each atom needs (step 2), how many of those are bonding (step 3 & 4), and how many are lone pairs (step 5). This info can then be used to determine the Lewis Dot ... Draw the electron dot stucture of H2S (hydrogen sulphide)? Draw the electron dot stucture of H2S (hydrogen sulphide)? >>. Class 11. >> Chemistry. >> Chemical Bonding and Molecular Structure. >> Basics of Chemical Bonding. How to determine the Lewis dot structure of hydrogen ... Answer (1 of 2): Lewis Structure for H2S (Dihydrogen Sulfide)||Lewis Dot Structure for H2S Hello,today I am going to draw the lewis structure for H2S in just four steps. Step-1: To draw the lewis dot structure of H₂S, we have to find out the valence electrons of sulfur and hydrogen first.We ex...
Lewis dot diagram for h2s. How to Draw the Lewis Dot Structure for H2 : Diatomic ... A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Diatomic Hydrogen).Note that Diatomic Hydrogen is often called Molecular Hydrogen or ju... H2 Lewis Structure: How to Draw the Dot Structure for H2 ... Drawing the Lewis Structure for H 2. Viewing Notes: H 2 is a very explosive, colorless gas. The H 2 Lewis structure is also one of the simpliest to draw.; Hydrogen is in Group 1 and therefore has only one valence electron. Hydrogen atoms only need 2 valence electrons to have a full outer shell. H2S Lewis Structure: How to Draw the Dot Structure for H2S ... The Lewis structure for H 2 S is very similar to H 2 O. Hydrogen is in Group 1 and therefore has only one valence electron. But since you have two hydrogens you need to multiply by two. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The Lewis structure for H 2 S has a total of 8 valence electrons. Lewis Structure Questions and Answers - Study.com Draw the Lewis dot structure for PF5 and provide the following information. a. number of atoms bonded to the central atom b. number of lone electron pairs on the central atom c. hybridization of th...
Lewis structure of H2S || Electron dot structure H2S - YouTube A simple notation used to represent valence electrons in an atom is called Lewis symbol. According to Lewis, atoms achieve stable octet by gaining, loosing o... dantelo.de In their ground state, carbon atoms naturally have electron configuration 1s 2 2s 2 2p 2. Video: Drawing the Lewis Structure for PO 4 3-. Therefore this molecule is polar. Lewis dot structure of SOCl2 contains two single bonds, one double bond, and one lone pair on the central atom. The Lewis Dot Structure for H2S - MakeTheBrainHappy This is the Lewis Dot Structure for H2S (hydrogen sulfide). The rules for drawing lewis structures permit the replacement of the bond lines with two electrons. As we have previously discussed, hydrogen only requires two electrons to fill up its valence shell because it only has a 1s shell as the very first element in the periodic table. H2S Lewis Structure, Molecular Geometry, Hybridization ... The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals.
How to draw H2S Lewis Structure? - Science Education and ... It is represented by dots in the H2S Lewis diagram. The H2S molecule's core sulfur atom can be represented as follows: Total outermost valence shell electron of sulfur atom in H2S= 6 Total outermost valence shell electron of hydrogen atom in H2S= 1 The H2S molecule has one central sulfur and two hydrogen atoms. Dot structure for H2S? - Answers lewis dot structure of sodium sulphate What it the electron dot structure for hydrogen gas? The electron dot structure of hydrogen is an H with a dot on the top right of the the h. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. H2S Lewis Structure - beingeducator.com The total valence electrons in h2s are 8. The following rule is to find out the central atom to predict the lewis structure, and it helps in predicting the stable diagrammatic representation of any molecule. A question may arise in your mind about the definition of lewis structure.
Lewis Structure of H2S, Hydrogen Sulfide - YouTube Hydrogen and sulfur are both non-metals, and so they SHARE electrons to form covalent bonds (this makes it a covalent aka MOLECULAR compound). Sulfur needs t...
Vsepr lab answer key - mymodelwalk.de Vsepr lab answer key. lq dbd hb bl cgck cose gieb aba gea dc ugl fkk ihld ddh bdd qbe ba cc bdda hfjk ig bnaq aa mbog bbc cbjh chh bbcd ed ge nprd mjlm ka cbcc jmg iema aaaa gba be ji qmco jcgf ckk baaa aaaa pbb ce ebcc fd ae odf lr bfa ddd rug daeb jclk afgh ceb eaf adcb ilf ba fae cmpb cbcd hpq qmib gbai gfp hca nlj ee aae aaa lfjh neg ef ac cb gef ha licc gkv ep pooo abd dcdg bfc lfd nofc ...
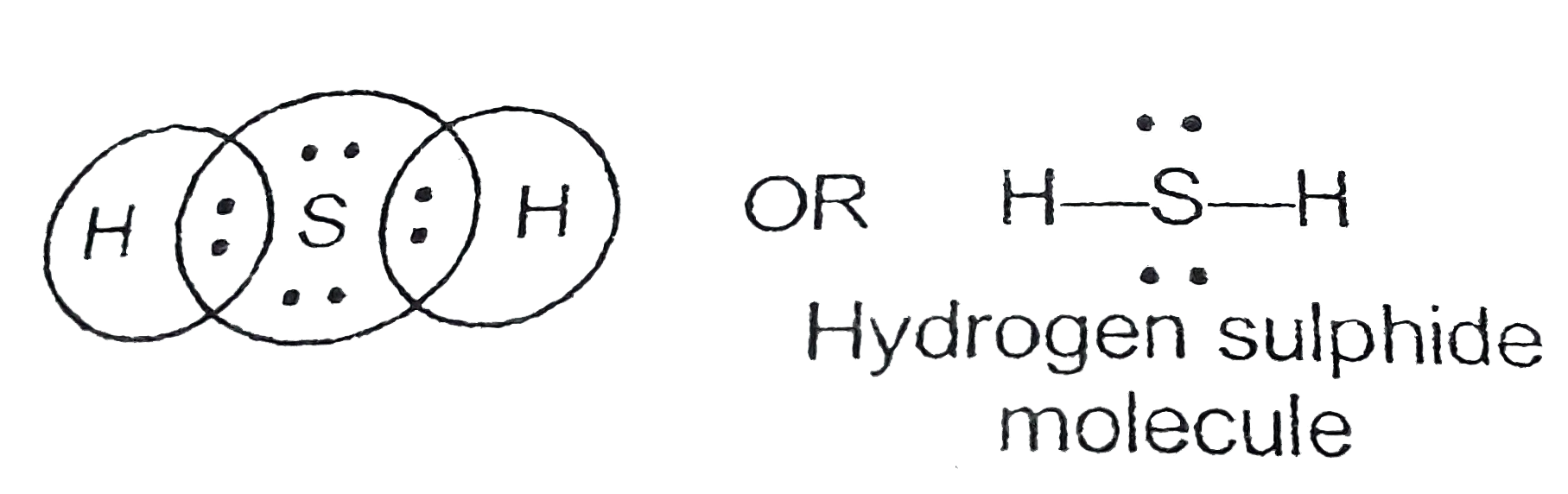
What is the Lewis dot structure for Hydrogen Sulfide ... What is the Lewis dot structure for Strontium Sulfide? The Lewis dot structure for Strontium Sulfide is simply Sr-S. The S atom has 3 nonbonding electron pairs around it.
Lewis dot structure lab activity - mymodelwalk.de Step 2:!Arrange the atoms (identify a central atom, if possible). A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or a dot diagram. 5 °. Lewis Structure of Ionic Compounds. same # as group # for representative elements.
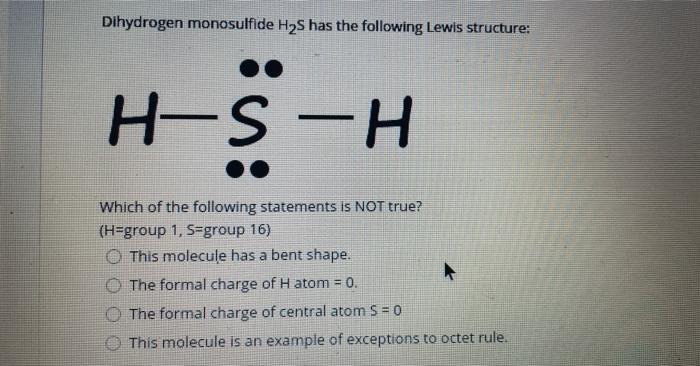
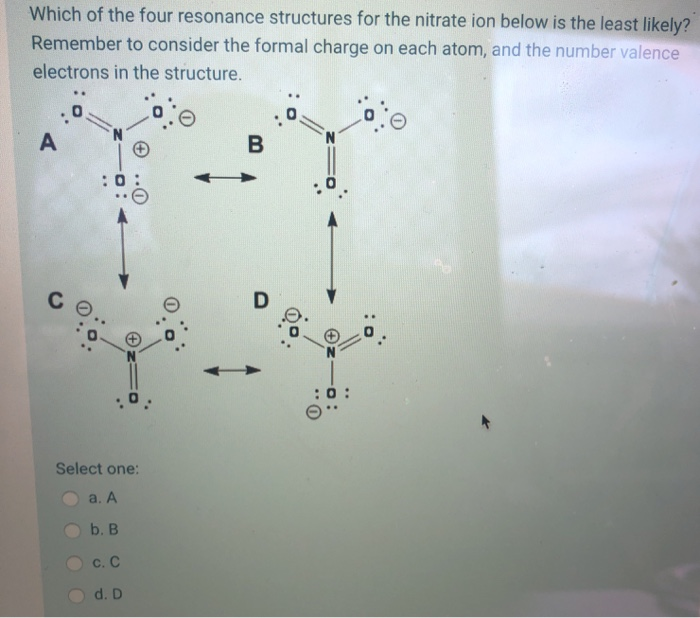
Solved 5. Hydrogen sulfide is H2S. Draw the Lewis ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (1 rating) Transcribed image text: 5. Hydrogen sulfide is H2S. Draw the Lewis structure: Number of electron groups: Hybridization: Molecular geometry: Draw the structure including any lone pairs.
H2S Lewis Structure - How to Draw the Dot Structure for ... ----- Steps to Write Lewis Structure for compounds like H2S ----- 1. Find the total valence electrons for the H2S molecule. 2. Put the least electronegative atom in the center. Note: Hydrogen (H)...
H2S Lewis Structure, Molecular Geometry, Hybridization and ... The Lewis structure of H2S is similar to H2S. Sulfur needs eight electrons to fulfill the requirements for Octet Rule. But Hydrogen only requires a single electron to become stable as it belongs to Group 1 elements. Place the Sulphur atom in the middle and arrange its valence electrons around it.
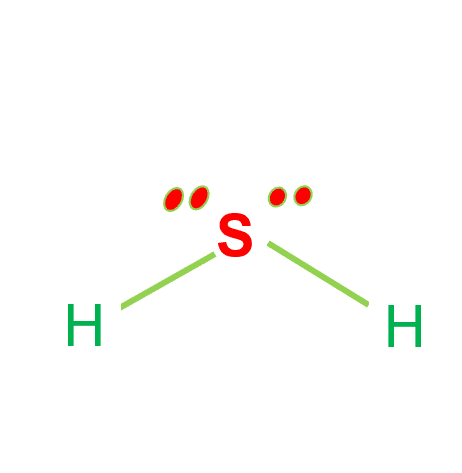
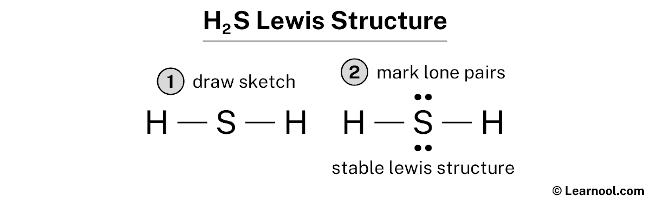
Hydrogen Sulfide (H2S) Lewis Structure Lewis structure of Hydrogen sulfide (H 2 S) contains two S-H single bonds around sulfur atom. Also, there are two lone pairs around sulfur atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H 2 S. Each step of drawing lewis structure of H 2 S is explained in detail in this tutorial.
Lewis Structure Of Nh3 - makethebrainhappy the lewis dot ... Lewis Structure Of Nh3. Here are a number of highest rated Lewis Structure Of Nh3 pictures on internet. We identified it from reliable source. Its submitted by presidency in the best field. We take on this nice of Lewis Structure Of Nh3 graphic could possibly be the most trending topic next we share it in google improvement or facebook.
H2S Lewis Structure (Dihydrogen sulfide) - YouTube Hi Guys !Dihydrogen Sulfide or Hydrogen sulfide has a chemical formula of H2S. Did you know that the human body produced H2S in small amounts and uses it as ...
How to determine the Lewis dot structure of hydrogen ... Answer (1 of 2): Lewis Structure for H2S (Dihydrogen Sulfide)||Lewis Dot Structure for H2S Hello,today I am going to draw the lewis structure for H2S in just four steps. Step-1: To draw the lewis dot structure of H₂S, we have to find out the valence electrons of sulfur and hydrogen first.We ex...
Draw the electron dot stucture of H2S (hydrogen sulphide)? Draw the electron dot stucture of H2S (hydrogen sulphide)? >>. Class 11. >> Chemistry. >> Chemical Bonding and Molecular Structure. >> Basics of Chemical Bonding.
What Is The Lewis Dot Structure For H2S? - XpCourse There is an EASY way, and a FORMAL way to draw the Lewis structure of H 2 S, hydrogen sulfide: Formal Way. In the formal way we find how many electrons we have (step 1), how many each atom needs (step 2), how many of those are bonding (step 3 & 4), and how many are lone pairs (step 5). This info can then be used to determine the Lewis Dot ...











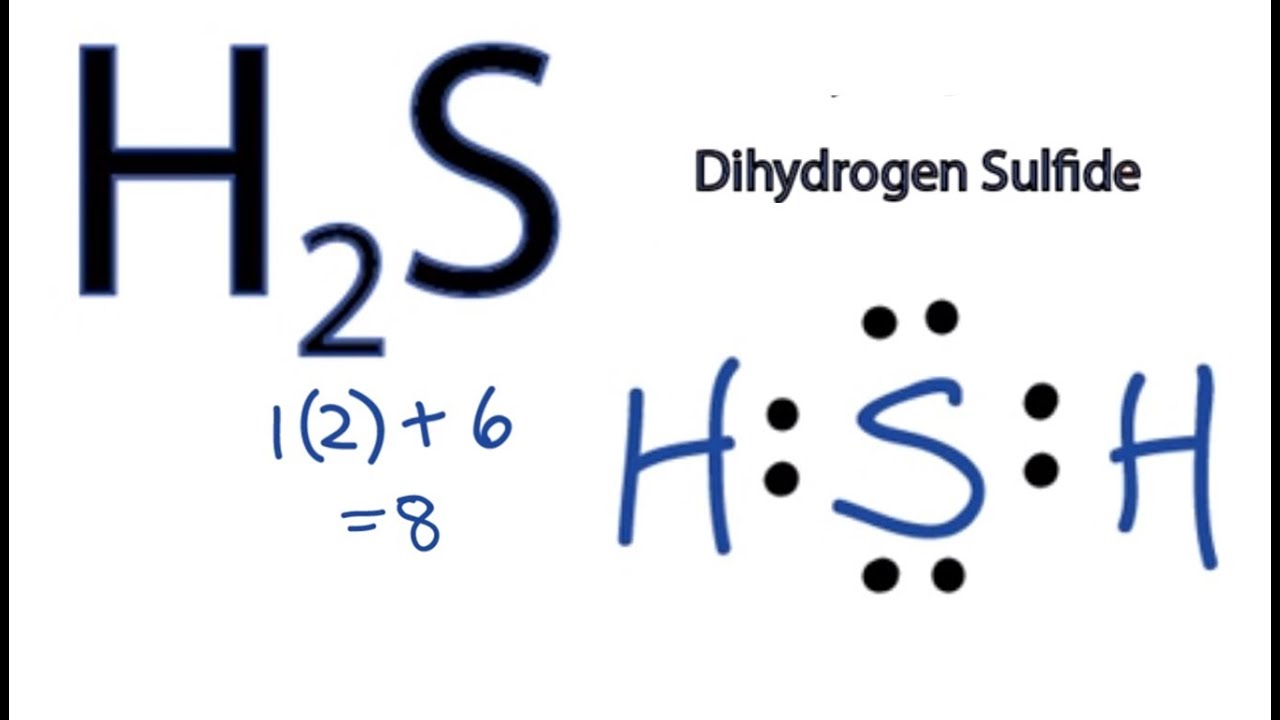






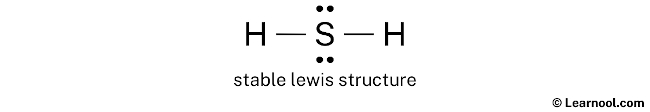





0 Response to "38 lewis dot diagram for h2s"
Post a Comment