38 bohr diagram for sulfur
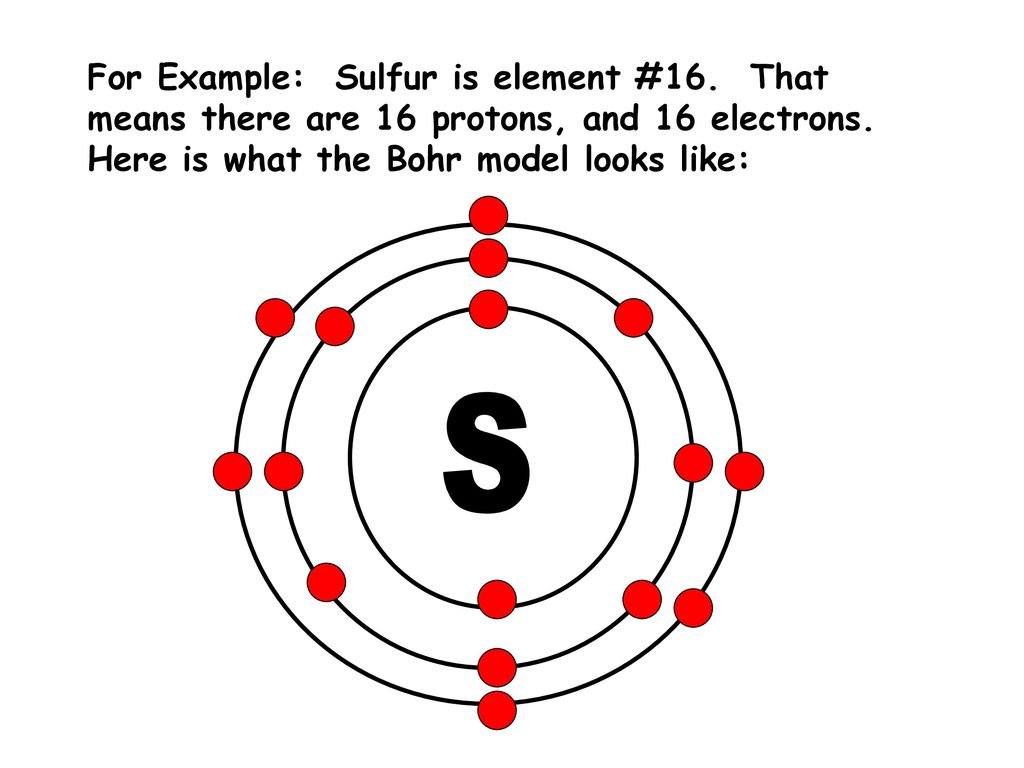
What is the Bohr diagram for sulfur? Sulfur has the atomic number 16; therefore, it has 16 protons and 16 electrons. The first energy level will fill up with two electrons. ... Now, since there are only six electrons left and the 3rd energy level has room for 18 electrons, the remaining six electrons will go in the 3rd energy level in the atom ... The Bohr Model of Potassium(K) has a nucleus that contains 20 neutrons and 19 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Potassium contains only 1 electron that also called valence electron.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
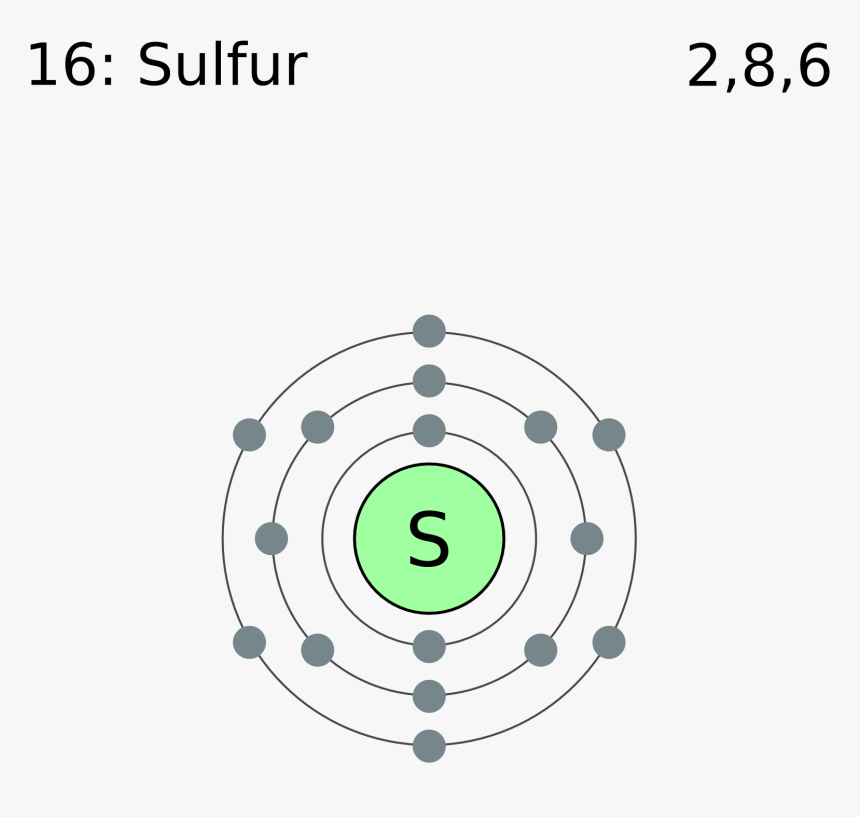
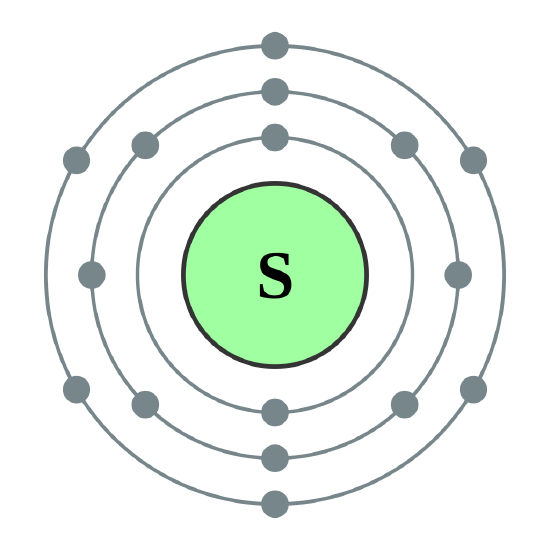
Bohr diagram for sulfur
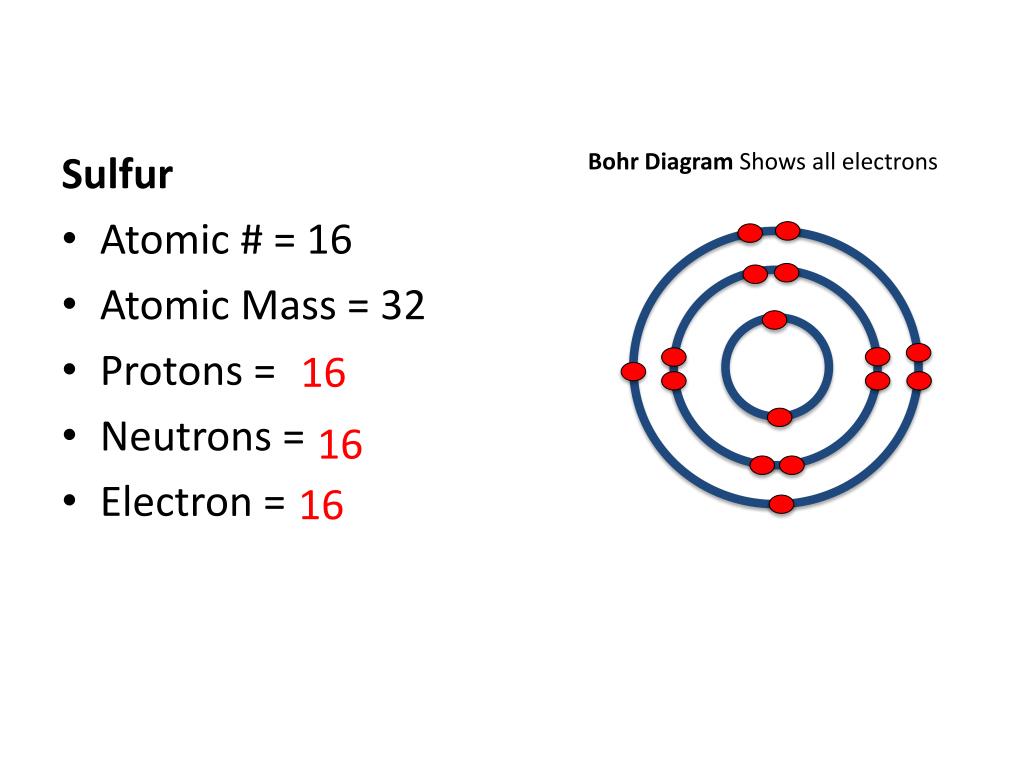
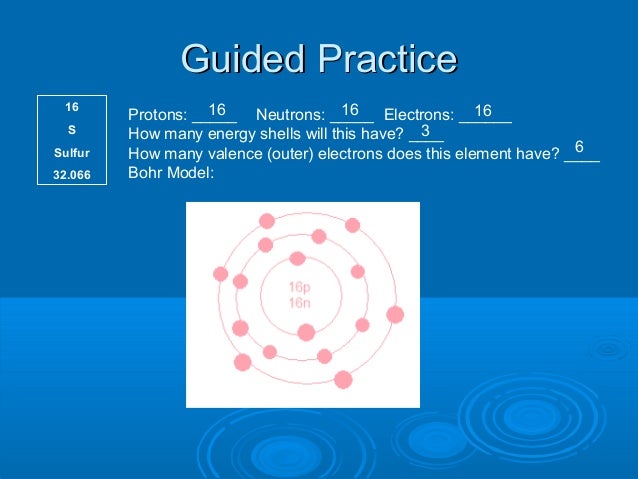
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons Bohr Diagram Sulfur Shows all electrons Atomic # 16 Atomic Mass — -32 Protons = I (O Neutrons Electron = 15. Calculate the missing information and then draw the Bohr Diagram and Lewis Structure for each element. Atomic # = 3 Mass # = 7 # of N = Atomic # 17 Mass # 35 Atomic # = 10 Mass # = 20 Atomic # = 2 Mass # 4 # of E = d Atomic # 12 Mass ... 14. Scientists use two types of diagrams to show the electron configuration for atoms. Follow your teacher's directions to complete the diagrams. Sulfur Atomic # = 16 Atomic Mass = 32 Protons = 16 Neutrons = 16 Electron = 16 15. Calculate the missing information and then draw the Bohr Diagram and Lewis Structure for each element. Mg Atomic ...
Bohr diagram for sulfur. Bohr Diagram - The Element Sulfur. The Element Sulfur. All About Sulfur! Bohr Diagram. Definition Conductivity Explanation based on Malleability Ductility 8. Draw a Bohr diagram of potassium and chlorine in an ionic bond. 9. Draw a Bohr diagram of one carbon atom and four hydrogen atoms in a covalent bond. 10. Draw a Bohr diagram of how sulfur and chlorine would bond 11. Draw a Bohr diagram of how sodium and fluorine would bond. 12. Bohr Diagram Hamle Rsd7 Org The sulfur atom has 16 protons 16 neutrons and 16 electrons in three different energy levels or orbits. Bohr diagram of sulfur. Bohr diagrams bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. The diagram depicts the atom as a positively charged nucleus surrounded ... sulfur Periodic Table • The Periodic Table organizes elements in different ways. • _____ are found on the left, _____ on the right, and metalloids in between. ... Draw Bohr diagrams of a magnesium atom bonding with fluorine atoms. What type of bonding occurs? Type of bond: _____ 14 . 4. Complete the following table.
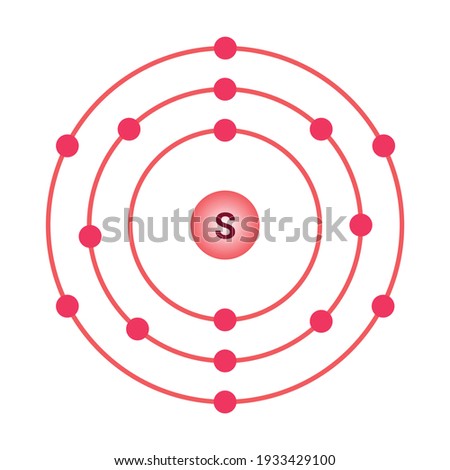
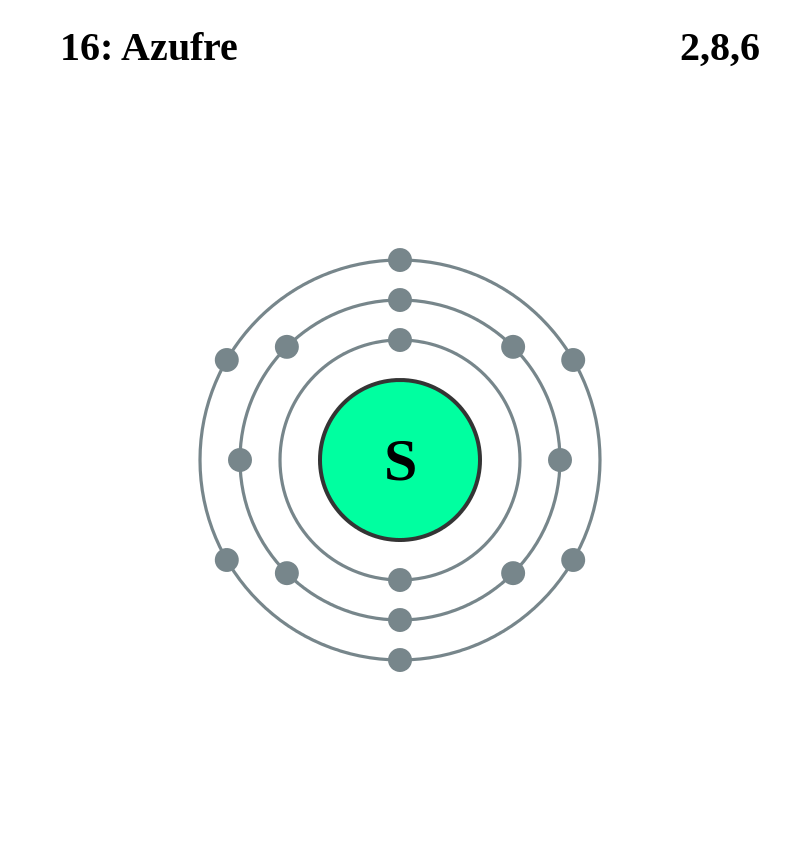
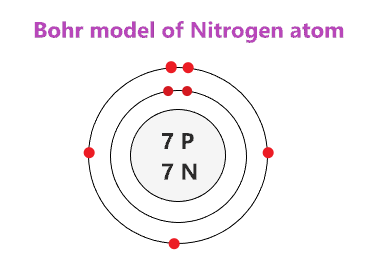
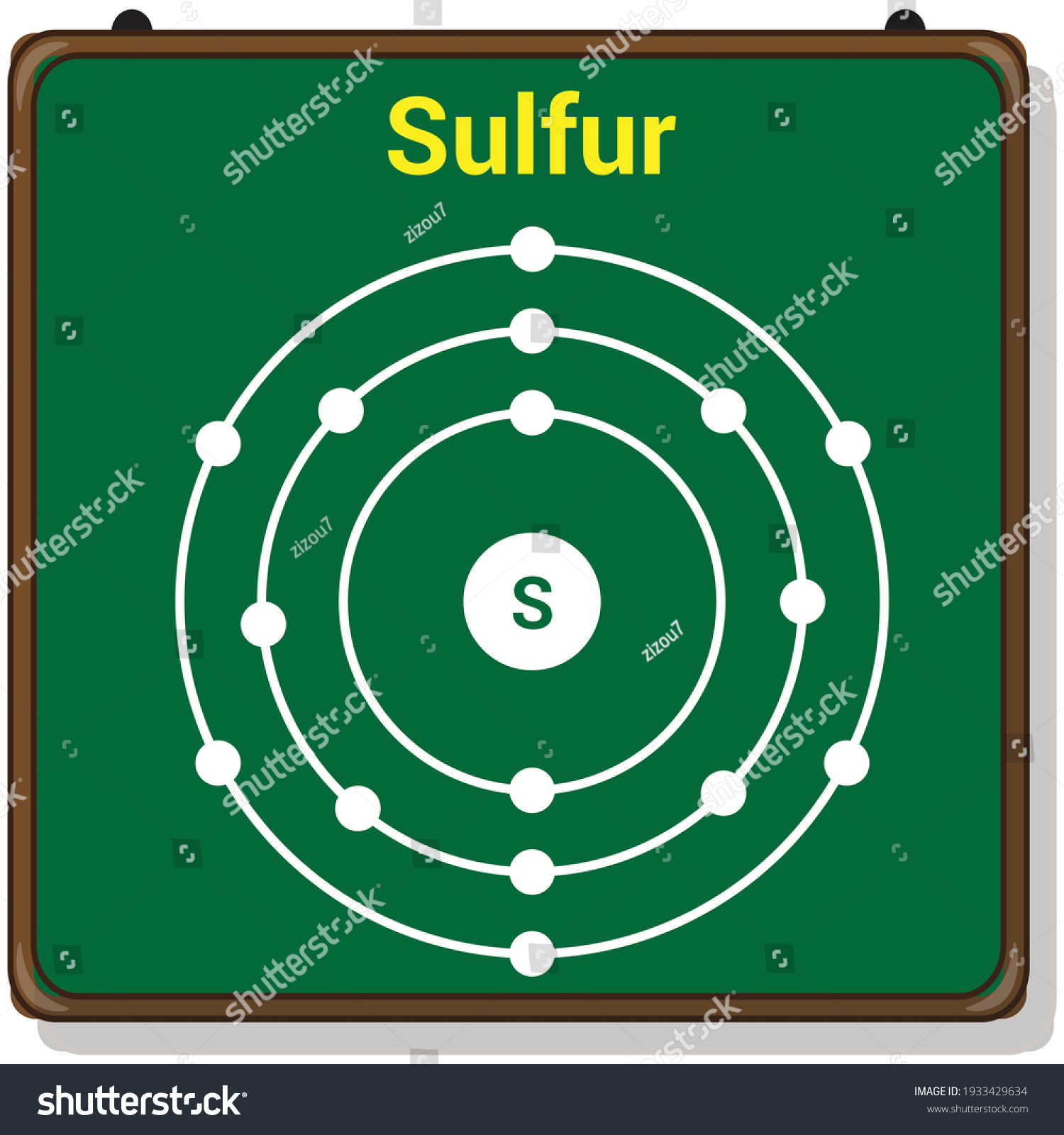
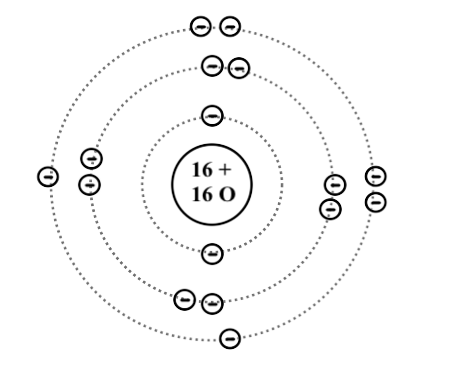
Bohr diagrams are used to introduce. The sulfur atom has 16 protons 16 neutrons and 16 electrons in three different energy levels or orbits. A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in 1913. 1st energy level 2 electrons max 2nd energy level 8 electrons max 3rd levels 18 ... Bohr model of Elements; 1: Bohr model of Hydrogen (H) 1: 2: Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr ... Sulfur Bohr Model Diagram. Sulfur at Chemical schematron.org Basic Information | Atomic Basic Information. Name: Sulfur Symbol: S [Bohr Model of Sulfur], Number of Energy Levels: 3. In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3,. The sphere n = 1 can accommodate two, the n = Model ... Sulfur / Sulphur has 2 electrons in its first shell, 8 in its second, 6 in its third.Check me out: http://www.chemistnate.com
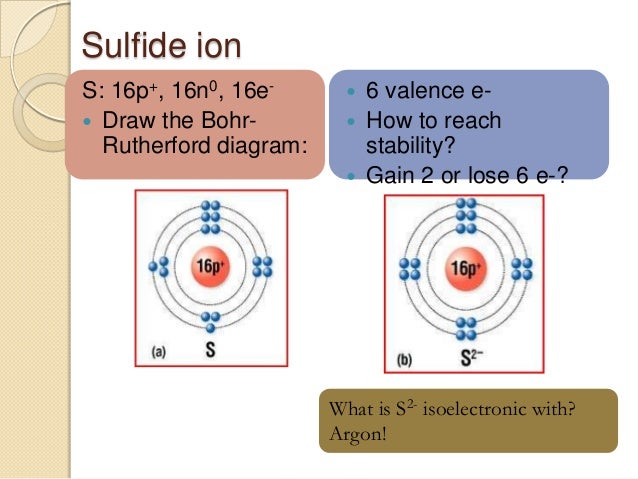
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Bohr Model and Lewis Dot Diagram Worksheet Answers or 50 Best Stock Lewis Dot Diagram for Sulfur Diagram Insp. Bohr model diagrams and lewis dot structures use the information provided for each element to draw bohr model diagrams. Basic atomic structure worksheet answers pdf. What is the charge. Lewis diagram for Sulfur. Click card to see definition 👆 ... Bohr diagram for Sodium. Lewis diagram for Sulfur. Carbon dioxide. CO2. Sulfur Hexafluoride. SF₆ ... 2021-11-06. Create. 2004-09-16. Sulfide (2-) is a divalent inorganic anion obtained by removal of both protons from hydrogen sulfide. It is a conjugate base of a hydrosulfide. Chemical groups containing the covalent sulfur bonds -S-. The sulfur atom can be bound to inorganic or organic moieties.

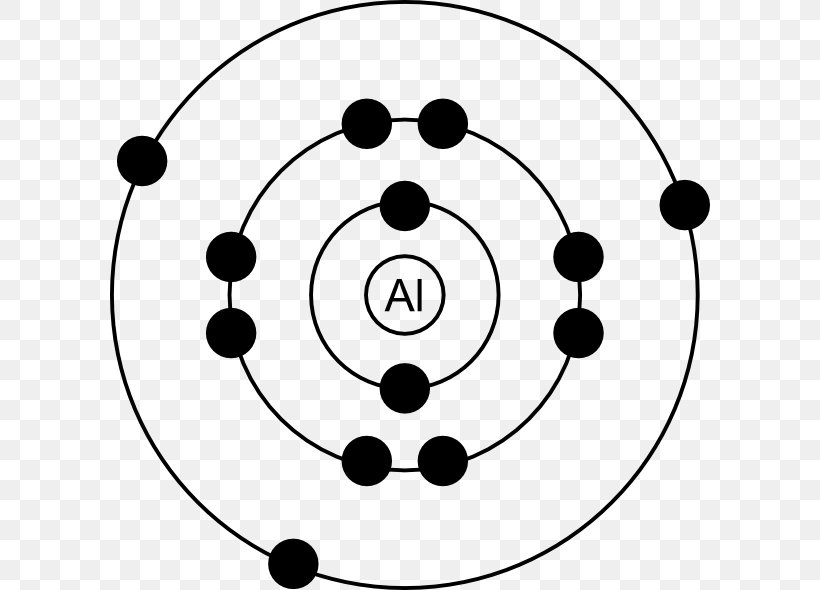
Bohr Model Electron Aluminium Lewis Structure Atom Png 600x590px Bohr Model Aluminium Aluminum Can Area Atom
The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. The bohr Rutherford diagram for oxygen has 8 protons and 8 ...
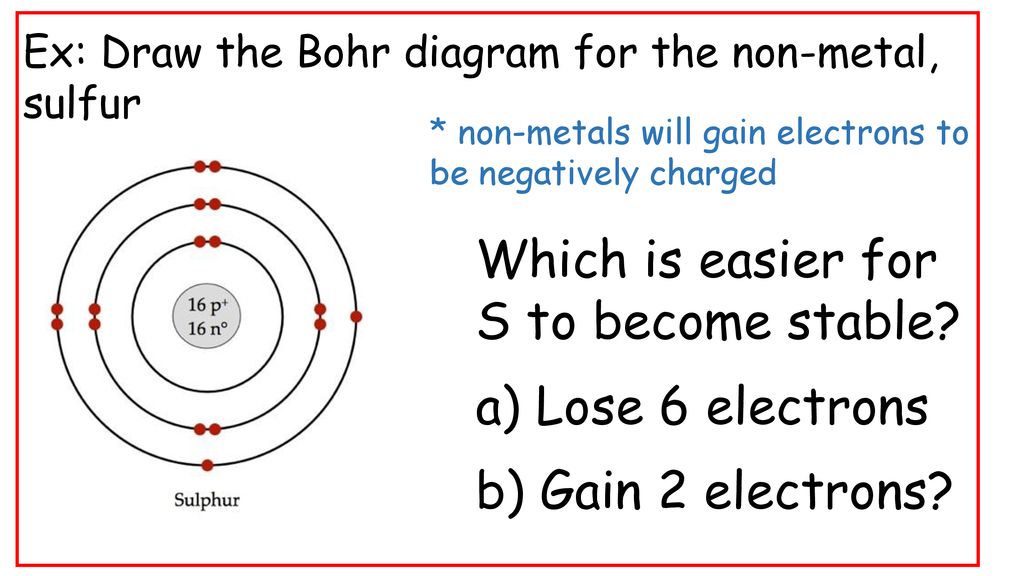
In 1922 Neils Bohr Won The Nobel Prize In Physics For His Understanding Of Atomic Structure His Theory States That Electrons Have Fixed Amounts Of Energy Ppt Download
All About Sulfur! Bohr Diagram. Picture. Powered by Create your own unique website with customizable templates. Get Started. A copper atom is a metal located in group period 4 of the Periodic Table of Elements. Its atomic symbol is Cu. Each atom has 29 protons and electrons. The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three ...
11+ Sulfur Bohr Diagram. Diagram of connection of the capacitive sensor, for the autolevel function, with an relay module. It is abundant, multivalent and nonmetallic. Sulphur atom - YouTube from i.ytimg.com Draw bohr, electron configuration notation and energy level diagrams for sulfur and vanadium. Each diagram also features the number…
5. Below is shown the PES spectrum of sulfur (atomic number = 16). a. Write the full electron configuration of sulfur. b. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) c. On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13).
Isotopes of Sulfur (click to see decay chain): 26 S 27 S 28 S 29 S 30 S 31 S 32 S 33 S 34 S 35 S 36 S 37 S 38 S 39 S 40 S 41 S 42 S 43 S 44 S 45 S 46 S 47 S 48 S 49 S : 35 S : Half-life: Fermion, 16p 19n: 87.51157407407 d: Spin 3/2 Parity 1: ... Click any isotope in diagram to see its data.
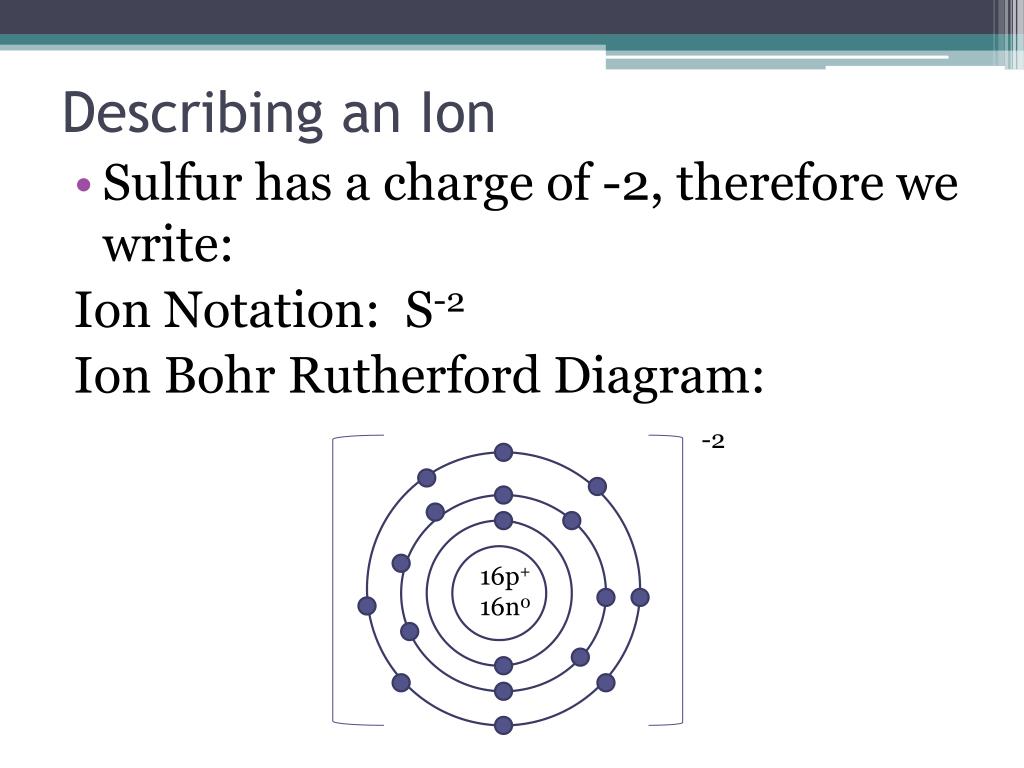
Find step-by-step Biology solutions and your answer to the following textbook question: Draw Bohr-Rutherford diagram for the sulfur-32 atom..
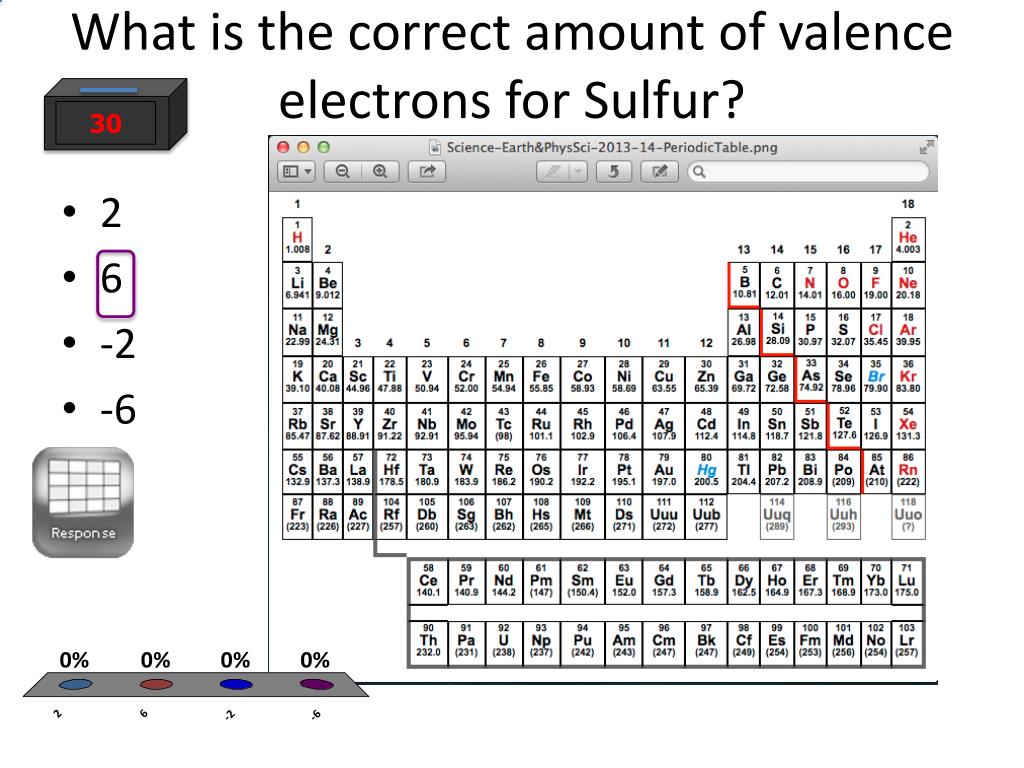
The Bohr Model of Sulfur(S) has a nucleus that contains 16 neutrons and 16 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sulfur contains 6 electrons that also called valence electrons.
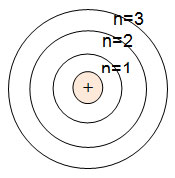
What is the Bohr diagram for sulfur? Bohr Model: In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n =1, 2, 3,....
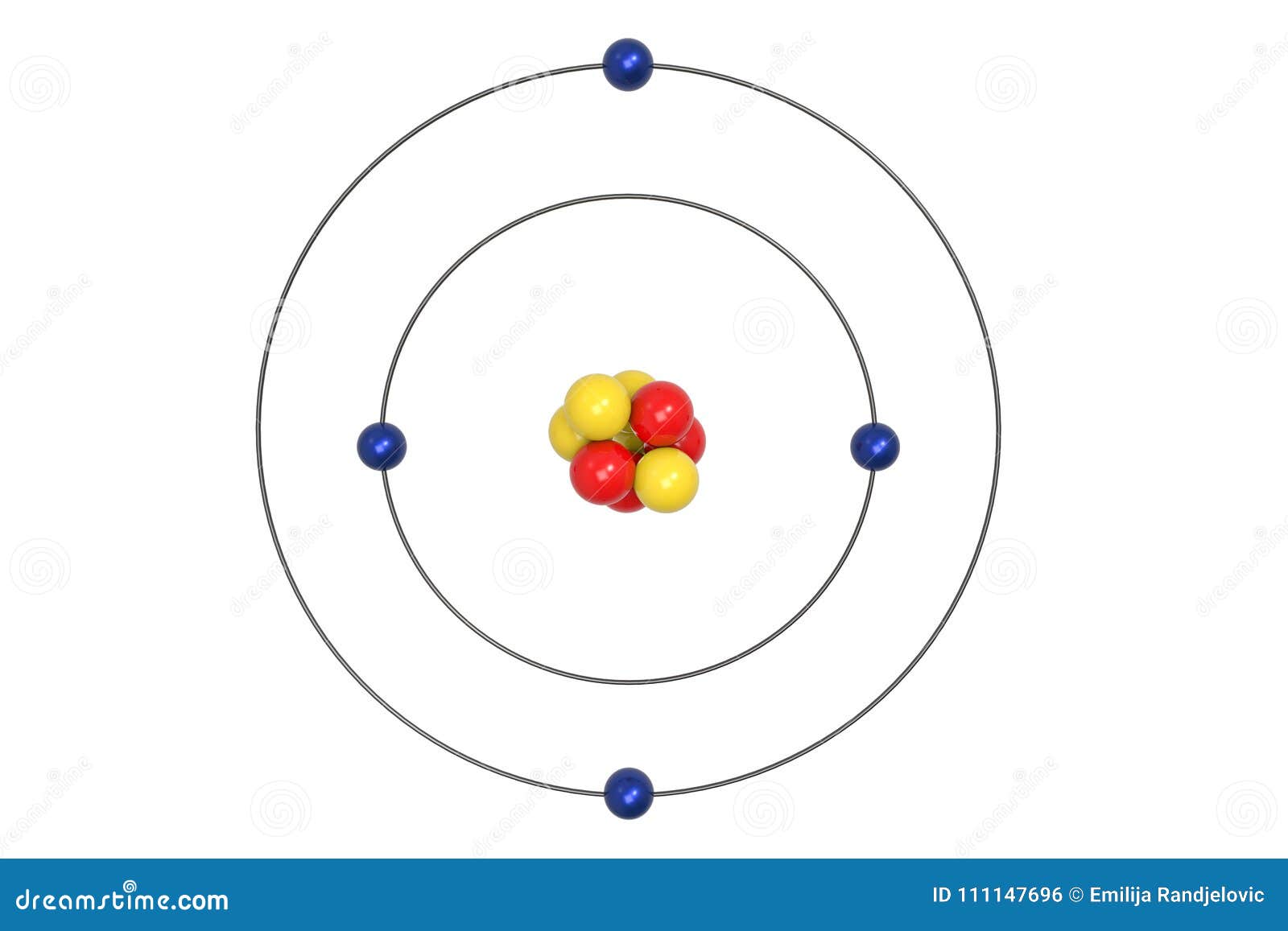
Beryllium Atom Bohr Model With Proton Neutron And Electron Stock Illustration Illustration Of Medicine Ball 111147696
Created with TouchCast https://itunes.apple.com/us/app/touchcast/id603258418For the interactive version visit:http://touchcast.com/msperrotti/bohr_model_sulfur
The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the third layer. This is because the atomic number of Potassium (K) is 19, therefore has 19 protons and 19 electrons. How to Make a Model of a Sulfur Atom.
Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.
14. Scientists use two types of diagrams to show the electron configuration for atoms. Follow your teacher's directions to complete the diagrams. Sulfur Atomic # = 16 Atomic Mass = 32 Protons = 16 Neutrons = 16 Electron = 16 15. Calculate the missing information and then draw the Bohr Diagram and Lewis Structure for each element. Mg Atomic ...
Bohr Diagram Sulfur Shows all electrons Atomic # 16 Atomic Mass — -32 Protons = I (O Neutrons Electron = 15. Calculate the missing information and then draw the Bohr Diagram and Lewis Structure for each element. Atomic # = 3 Mass # = 7 # of N = Atomic # 17 Mass # 35 Atomic # = 10 Mass # = 20 Atomic # = 2 Mass # 4 # of E = d Atomic # 12 Mass ...
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons

Why And How Does The Atomic Radius Change When An Atom Becomes A Cation Or An Anion A Element To Cation B Element To Anion Study Com
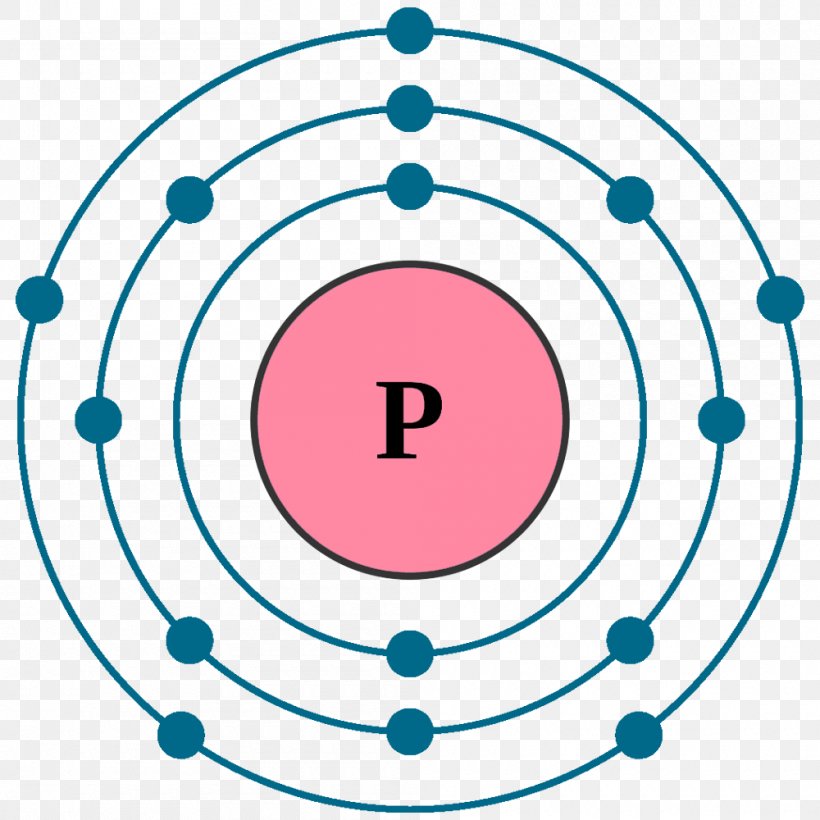
Electron Configuration Noble Gas Atom Bohr Model Png 1000x1000px Electron Configuration Argon Atom Atomic Orbital Bohr
Solved Draw The Bohr Model Of Sulfur Atom On Your Drawing Label Indicate Valence And Core Electrons Course Hero




:max_bytes(150000):strip_icc()/Selenium-58b601fd3df78cdcd83d2a90.jpg)







0 Response to "38 bohr diagram for sulfur"
Post a Comment