38 energy diagram for exothermic reaction
ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Solutions for Chapter 9 Problem 39EP: Sketch an energy diagram graph representing an exothermic reaction, and label the following.a. Average energy of reactantsb. Average energy of productsc. Activation energyd. Amount of energy liberated during the reaction…

Energy diagram for exothermic reaction
Energy changes accompany chemical reactions. Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (E a), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1. Endothermic and exothermic reactions are ... The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
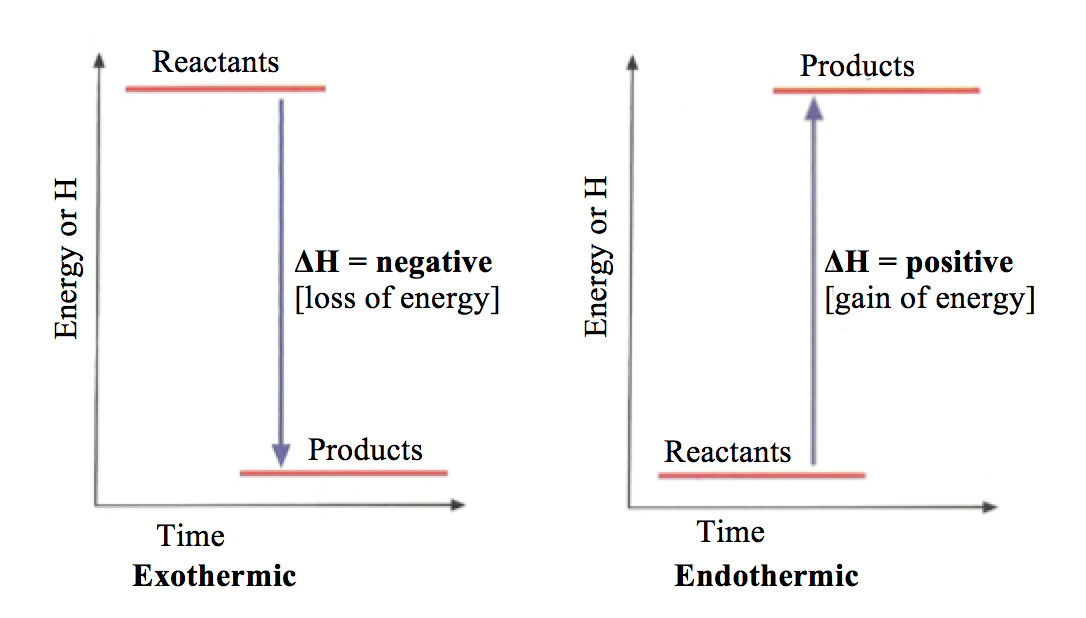
Energy diagram for exothermic reaction. 43 Given the potential energy diagram for a chemical reaction: Which statement correctly describes the energy changes that occur in the forward reaction? (1) The activation energy is 10. kJ and the reaction is endothermic. (2) The activation energy is 10. kJ and the reaction is exothermic. Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy. Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0). Exothermic Diagram. Energy released in bond making. Activation Energy. Energy used in bond. breaking. Exothermic - More energy is released when the products where formed than energy was used to break bonds in the reactants. Therefore, a net release of energy.
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. Energy level diagram for an exothermic chemical reaction without showing the activation energy.It could also be seen as quite exothermic with a highly unlikely zero activation energy, but reactions between two ions of opposite charge usually has a very low activation energy. Could usually . has a very low activation energy. The reaction shown by the second diagram is more exothermic. There is a greater difference in energy between the reactants and products. The green arrow is longer. 8:40Exothermic Energy Diagram: Activation Energy, Transition States and ... a reaction mechanism diagram which ...Apr 9, 2019 · Uploaded by Melissa Maribel
An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. Exothermic ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams.... Exothermic reactions uses/loses energy/heat during the reaction, so when drawing the energy diagram, you want the reactants on the graph to be higher than the ... Jul 9, 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
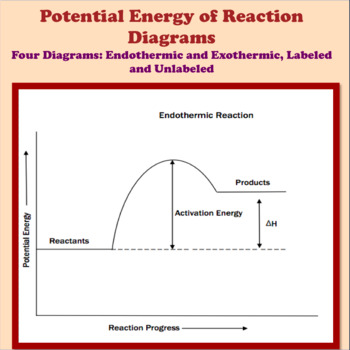
Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
The Course Of A Reaction Figure 13 4 Plots The Course Of A Reaction The Initial Average Energy Of The Reactants Is Indicated At The Left Side Of Each Graph If Molecules Are To Collide Effectively They Must Have More Than The Average Energy They Must Have
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ...
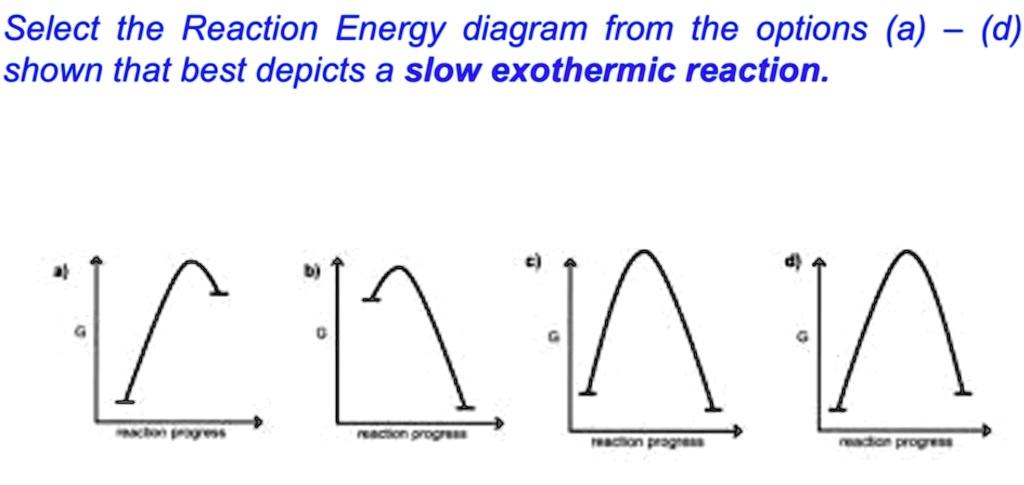
Solved Select The Reaction Energy Diagram From The Options A D Shown That Best Depicts A Slow Exothermic Reaction
Energy Diagrams Activation energy is the energy a reaction needs to get started. At the bottom of the page are two energy diagrams—one for an exothermic reaction and one for an endothermic reaction. Follow the directions below to label the energy diagrams. 1. In an exothermic reaction, the chemical energy of the reactants is greater than
Solved A Student Made A Sketch Of A Potential Energy Diagram To Represent An Exothermic Reaction Explain Using Complete Sentences Why The Diagra Course Hero
Energy Diagram: The diagram which indicates the number of steps in which the reaction gets completed and the energy required for each step can be shown by plotting graph of rate of reaction versus ...
This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram. However some energy is required for A to convert to B. This energy is called the activation energy or E act. Sometimes this is also called the activation barrier.
Question: Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential ...
FIGURE 13.4 Energy changes during reaction: (a) an endothermic reaction; (b) an exothermic reaction. This picture of a reaction is analogous to riding a bicycle over a mountain pass. The activation energy of the reaction is comparable to the energy needed to pedal to the top of the pass.
A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed. Shows whether a reaction is exothermic. Figure shows the energy level diagram for the reaction between methane and oxygen.
Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof. Endothermic vs. exothermic reactions.
An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to activate the reaction, and the ending energy. An enthalpy ...
A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below.
Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs?
You can start with a generic potential energy diagram for an exothermic reaction.. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed.. So, the activation energy is the minimum amount of energy required for a reaction to take place.
Exothermic reaction In an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings. Endothermic Reactions. Endothermic reactions are reactions that require external energy, usually in the form of heat, for the ...
4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram Igcse Chemistry Revision Help
diagram. The vertical axis on this diagram represents the energy level and the horizontal axis represents the progress of the reaction from reactants to products. Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between
Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction.
Energy changes accompany chemical reactions. Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (E a), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1. Endothermic and exothermic reactions are ...
Igcse Chemistry 4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram
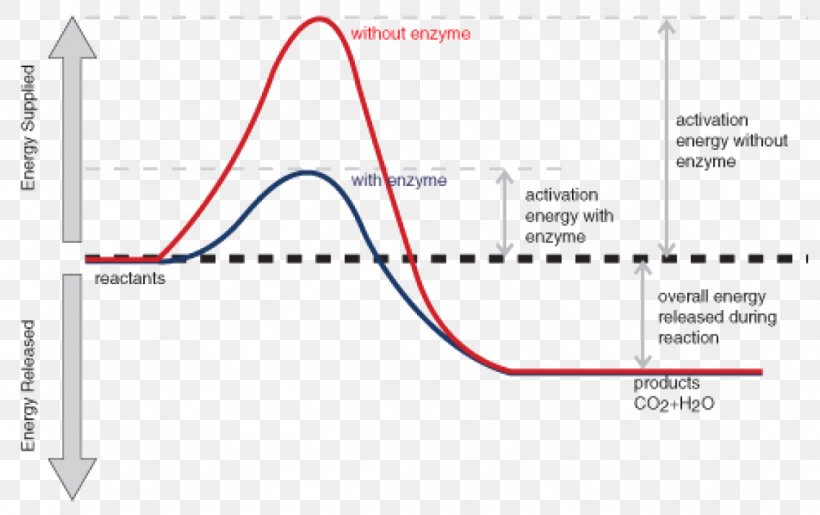
Activation Energy Chemical Reaction Enzyme Exothermic Reaction Png 1111x698px Activation Energy Activation Adenosine Triphosphate Anabolism Area

Question Video Identifying Steps In A Reaction Profile Diagram For A Two Step Chemical Reaction Nagwa

Draw An Energy Diagram For An Exothermic Reaction Label The Activation Enthalpy And The Change In Enthalpy Delta H On The Diagram Study Com















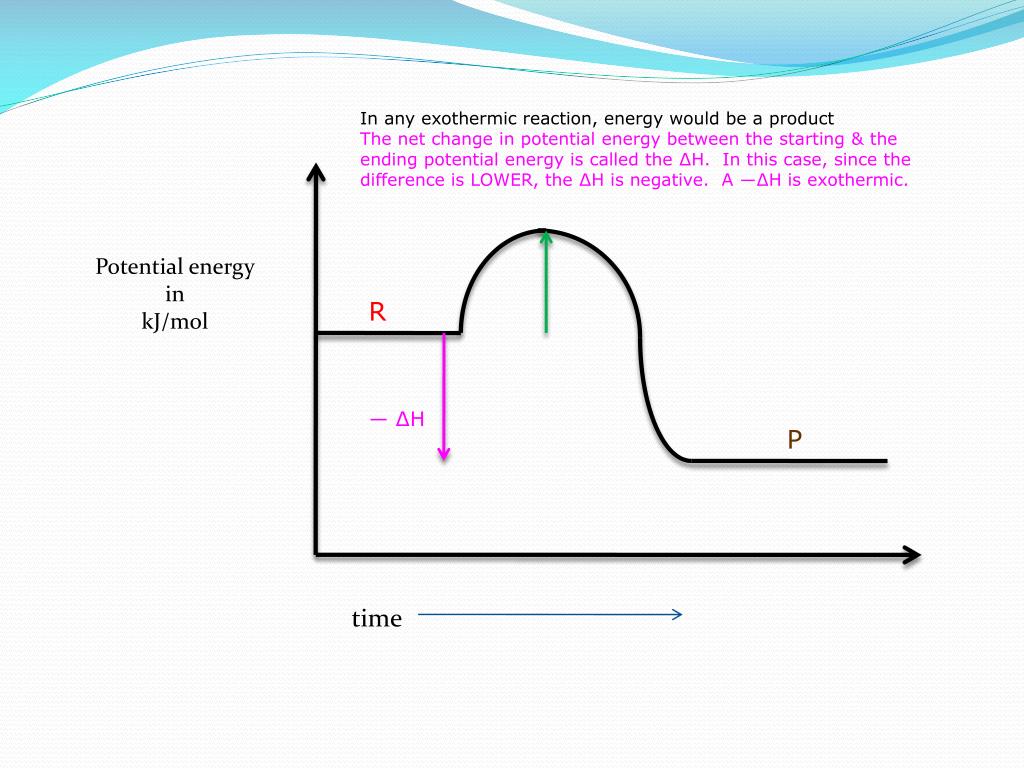


0 Response to "38 energy diagram for exothermic reaction"
Post a Comment