39 maximum solubility phase diagram
In the following Iron-Carbon phase diagram, we can see that the maximum concentration of Carbon is 6.67%. As for solubility of carbon is concerned, amount of cabon allowed is defined by the crystal structure, which in this case is about 25% quantity wise. The maximum solubility of excess Te decreases below this temperature due to the retro- grade slope of the T-x diagram, and the concentra- tion of excess Second phase defects are eliminated by post-growth stoichiometric annealing and the semi-insulating CdTe is prepared by re-annealing under Te or...
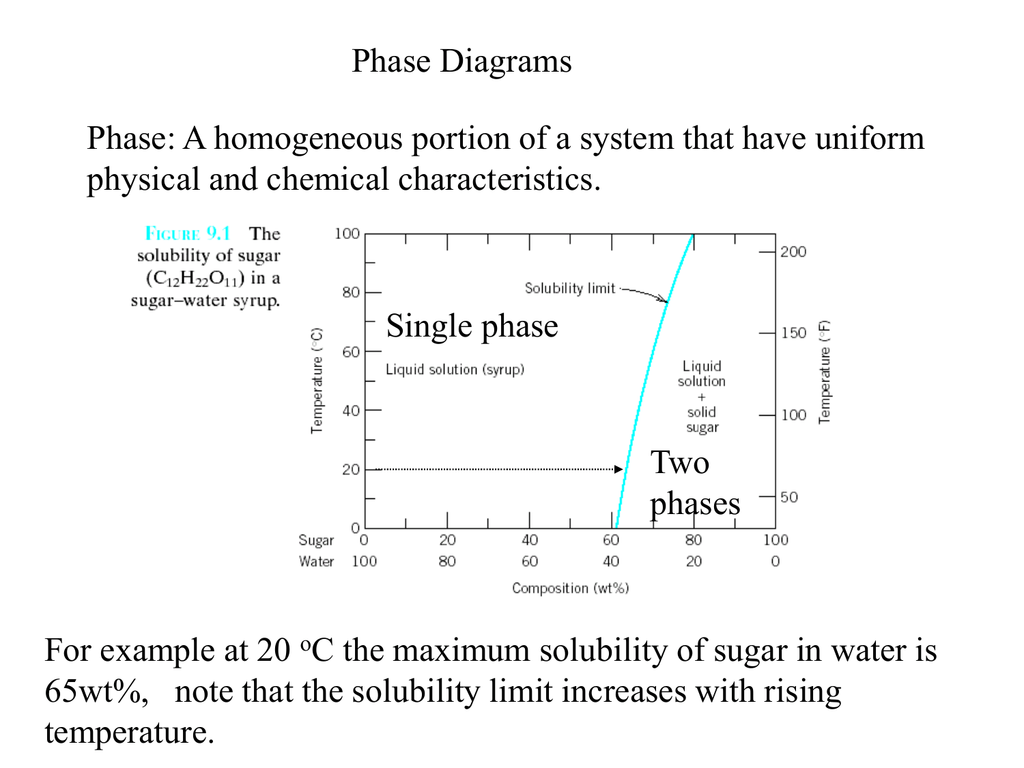
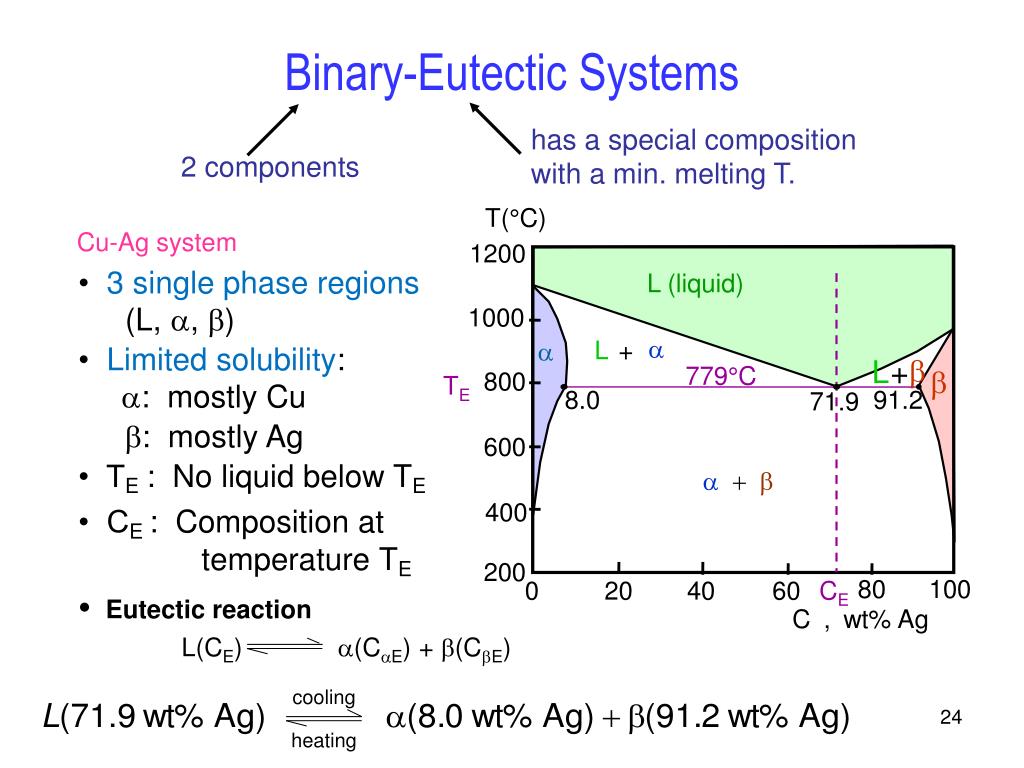
} Phase diagrams allows to predict phase transformations which occur during temperature change (e.g. upon cooling). • Solubility Limit: Max concentration for which only a solution occurs (remember HUME solubility at room (20°C) temperature (C = 2wt%) and maximum solid solubility at eutectic...
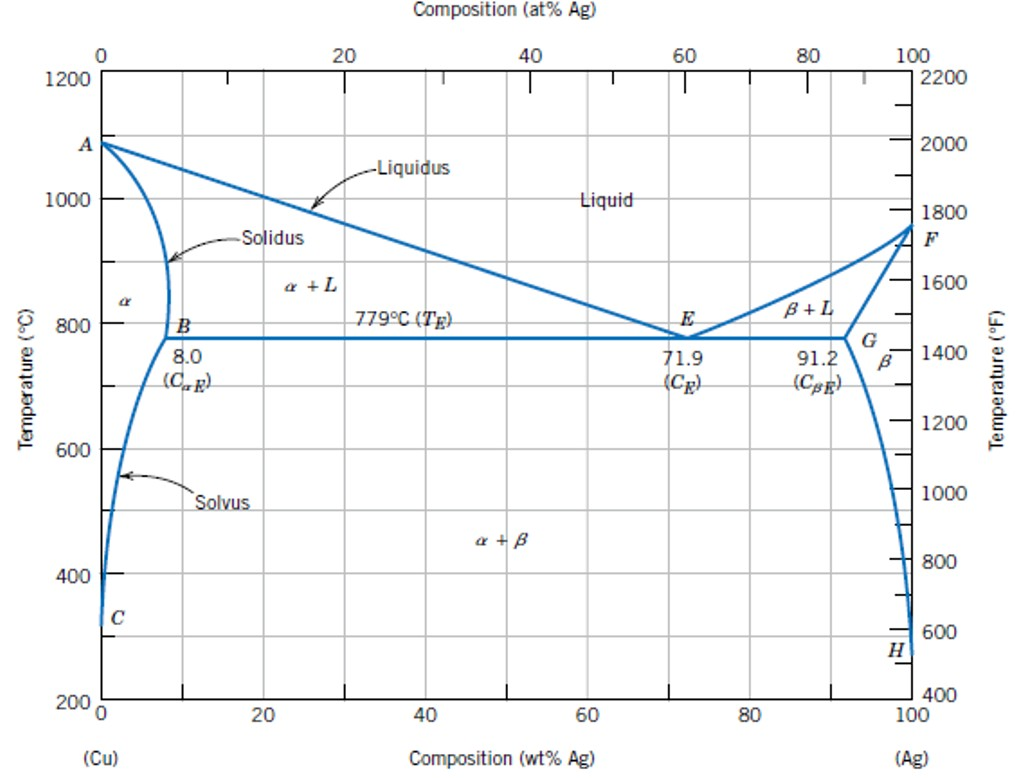
Maximum solubility phase diagram
The phase diagram is the 50 °C solubility isotherm for ammonia - phosphoric acid mixtures. Four solid phases appear on this isotherm: NH4H2PO4·H3PO4 The solubility of calcium hydroxide in water is quite low and it is decreasing with increasing temperature. This phase diagram covers the... К оглавлению: Другие диаграммы (Others phase diargams). Nb-Ni (Niobium-Nickel) P. Nash and A. Nash The maximum solubility of Ni in (Nb) is 4.5 at.% Alloy Phase Diagrams, 7(2), Apr 1986. Complete evaluation contains 4 figures, 2 tables, and 71 references. The solubility diagrams obtained provide complementary information for the Eh-pH diagrams: they define the limit of the domain of stability for each phase at different iron Infrared spectrum, solubility, and ternary solubility diagram indicated that dl-thiazolidine-4-carboxylic acid is a conglomerate.
Maximum solubility phase diagram. Phase diagrams provide some fundamental knowledge of what the equilibrium structure of a metallic (or ceramic) alloy is, as a function of temperature and composition. The real structure may not be the equilibrium one, but equilibrium gives a starting point from which other (non-equilibrium) structures... The phase diagrams are calculated based on the most up-to-date optimized parameters. The thermodynamic model parameters for all the Zn using thermal analysis and microscopic inspection. Accordingly, the maximum limited solubility of Mg in Zn was determined as 0.3 at.% at 673 K. Laves... Review of Phase Transformation. Diagrams. Solution and Solubility. Example: Solubility of salt in water There exists a maximum amount of salt that can be The same concepts apply to solids: solid solution, saturation, solubility, precipitation. Phase Diagrams. Pressure Super-critical fluid. A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically...
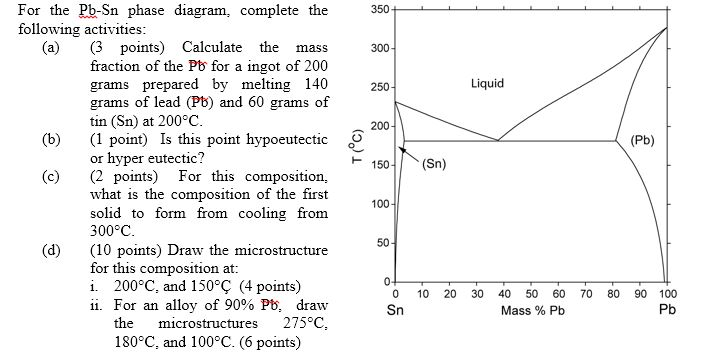
Suppose the maximum solubility of lead in tin is 5% Pb. whether or not we have a single or two-phase alloy depends on the temperature of the alloy. Which of the following is not a condition of unlimited solubility? Pmax - maximum # of phase(s). For Cigar shape diagram three parameters are available: 1. Phase presents: just locate the Temperature-Composition point and determine the. Partially solubility (eutectic diagram). Single phase Double phase Three phases. What is an equilibrium phase diagram? Experimental determination of a phase diagram Thermal a solid (α) state - Solvus: the line which represents the limit of solid solubility of a phase. components n: number of variables which can vary (temperature, pressure, volume) φ: maximum number of stable... interpretation of phase, simple binary phase diagrams, and development of equilibrium. microstructures upon cooling. 9.2 Solubility Limit ™ Solubility limit is the maximum concentration of solute atoms that may dissolve in the solvent to. form a solid solution at a specific temperature.
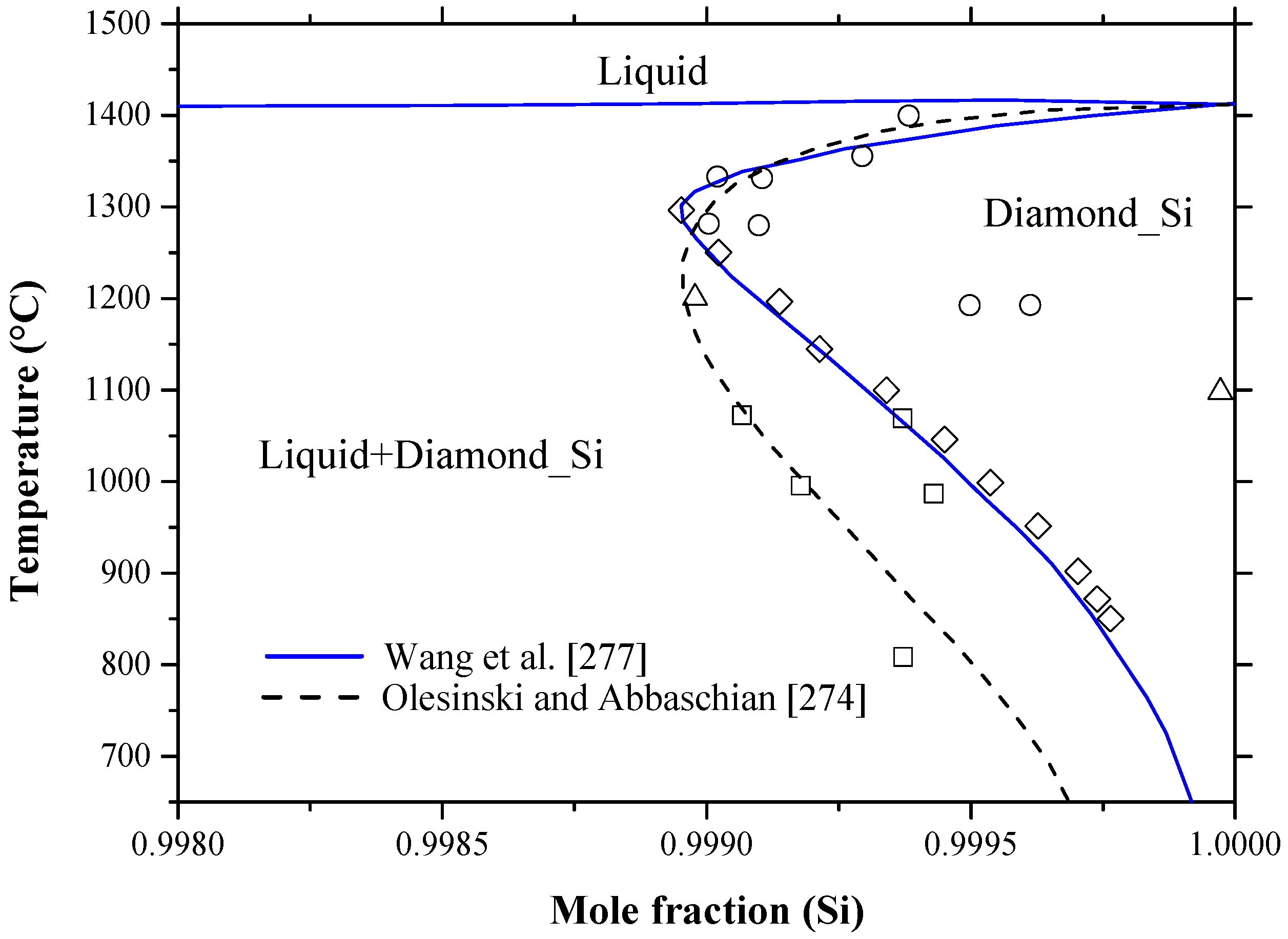
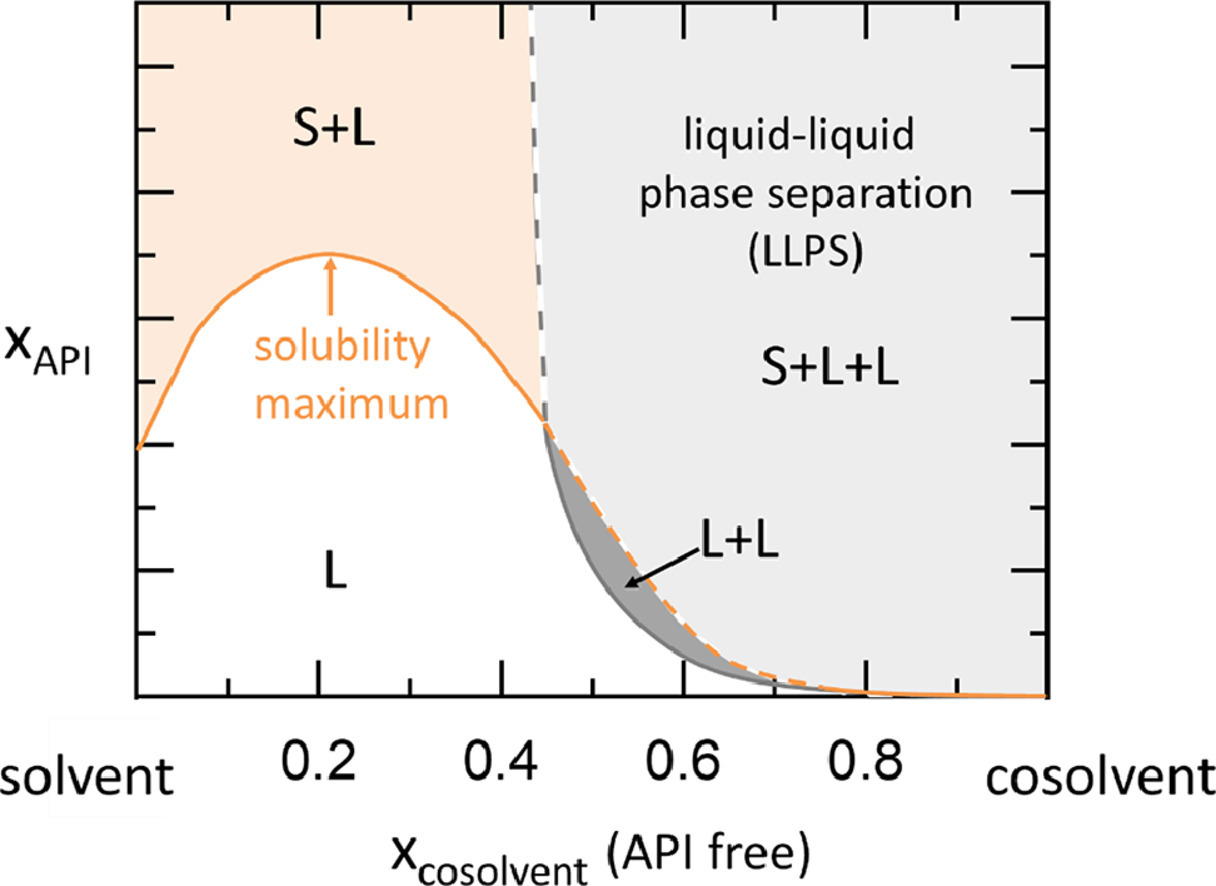
Phase Diagram. called critical solution temperature (CST)or consolute temperature (CT) and corresponding composition is called consolute From the graph, obtained maximum solubility temp and its corresponding composition; those will be critical solution temperature(CST) and con solute... The data for solubility maximum for H2O and CH4 are not available. From the Fig. 2 (b) and (c), we see the reason why it is dicult to perform these in We propose the Frenkel line serve as a predictive tool to locate the solubility maxima on the phase diagram. This provides a useful guide for future... However, the calculated phase diagram [155] showed the maximum solubility of B in Si as 1.6 at % B at 1385 °C (as opposed to 3.5 at % at 1362 °C [142]), trying to fit their calculations with the experimental points of [146,148]. The enthalpies and entropies of formation of the intermetallic compounds values... • Solubility Limit: Maximum concentration for which only a single phase solution exists. Question: What is the. § A phase diagram in which the components display unlimited solid solubility. © 2011 Cengage Learning Engineering. All Rights Reserved.
With phase diagram, it is possible to calculate the composition and amount of one phase to the The maximum temperature at which the two phase region exists is called the critical solution However, if the substance is soluble in both liquids, the mutual solubility increases as the upper consolute...
h Phase diagrams (constitutional diagrams) exhibit pictorial representation of relationships and product description. h Solubility Limit of a component in a phase: the maximum amount of component that can be dissolved in (e.g. alcohol has unlimited solubility in water, sugar has a limited...
Phase diagrams provide a convenient way of representing which state of aggregation (phase or phases) is stable for a particular set of conditions. Solubility limit - for almost all alloy systems, at a specific temperature, a maximum of solute atoms can dissolve in solvent phase to form a solid...
Thinking of binary phase diagrams in terms of solubility limits.
10 Projected and mixed phase diagrams. 11 Direction of phase boundaries. 12 Sharp and gradual phase transformations. force for the formation of a new phase 7.8 Partitionless transformation under local equilibrium 7.9 Activation energy for a uctuation 7.10 Ternary systems 7.11 Solubility product.
— Various phases that appear on the Iron-Carbon equilibrium phase diagram are as under — Maximum solubility is 2.0 % C at 1130°C. — High formability, most of heat treatments begin. with this single phase. — It is normally not stable at room temperature.
FactSage 8.1 - List of Stored Phase Diagrams (7811). FACT Databases. Click on a system to display the phase diagram.
Structure, analysis, Phase composition, Binary phase diagrams. Из урока. Phase diagrams with polymorphous elements and compounds. Module 5 focuses on phase diagrams for polymorphic elements.
In general, the maximum solubility of the individual fission product species in the fuel matrix will be reduced in the presence of additional constituents. Formation of adducts between LiCl and c-caprolactam or pyrro-lidone having 1 4 composition was observed by solubility and phase diagram...
A binary phase diagram is a temperature - composition map which indicates the equilibrium phases present at a given temperature and composition. Binary solutions with unlimited solubility (I). Let's construct a binary phase diagram for the simplest case: A and B components are mutually soluble in...
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Phase diagrams illustrate the variations between the states of matter of elements or compounds as they relate to pressure and temperatures.
Introduction Solubility Limits Phases Phase Equilibrium Interpretation of Phase Diagrams Binary Isomorphous Systems (Cu-Ni) 100 Temperature (°C) Maximum concentration for which only a single phase solution exists. Solubility Limit 80 60 L 40 (liquid solution i.e., syrup) 20 L (liquid) +.
The solubility diagrams obtained provide complementary information for the Eh-pH diagrams: they define the limit of the domain of stability for each phase at different iron Infrared spectrum, solubility, and ternary solubility diagram indicated that dl-thiazolidine-4-carboxylic acid is a conglomerate.
К оглавлению: Другие диаграммы (Others phase diargams). Nb-Ni (Niobium-Nickel) P. Nash and A. Nash The maximum solubility of Ni in (Nb) is 4.5 at.% Alloy Phase Diagrams, 7(2), Apr 1986. Complete evaluation contains 4 figures, 2 tables, and 71 references.
The phase diagram is the 50 °C solubility isotherm for ammonia - phosphoric acid mixtures. Four solid phases appear on this isotherm: NH4H2PO4·H3PO4 The solubility of calcium hydroxide in water is quite low and it is decreasing with increasing temperature. This phase diagram covers the...













0 Response to "39 maximum solubility phase diagram"
Post a Comment