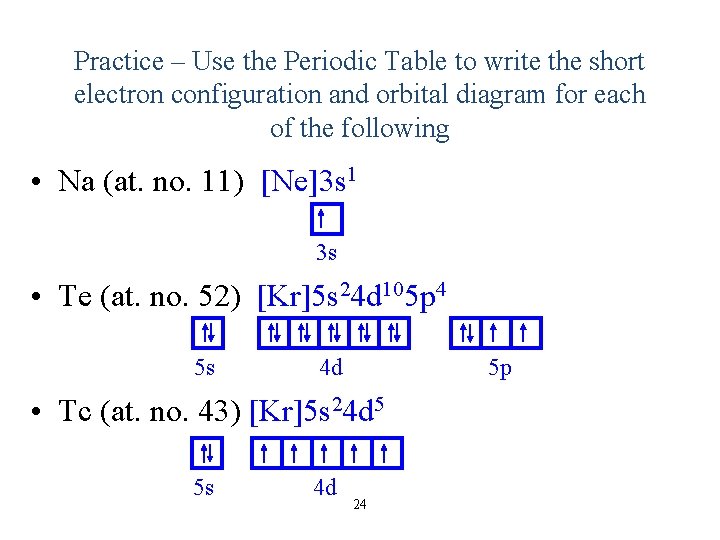
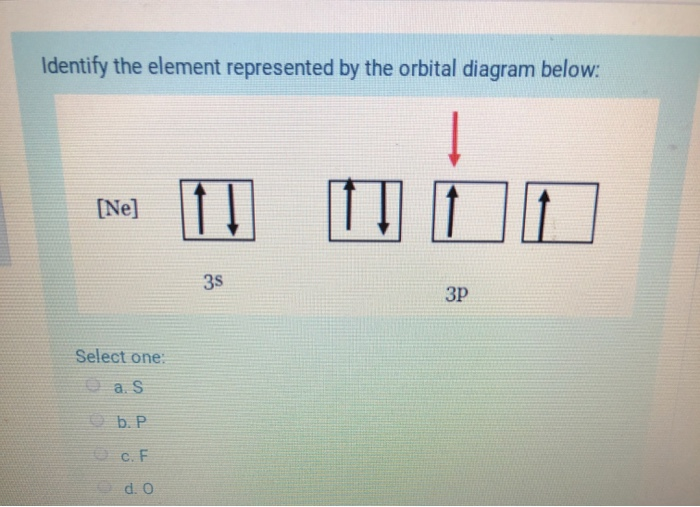
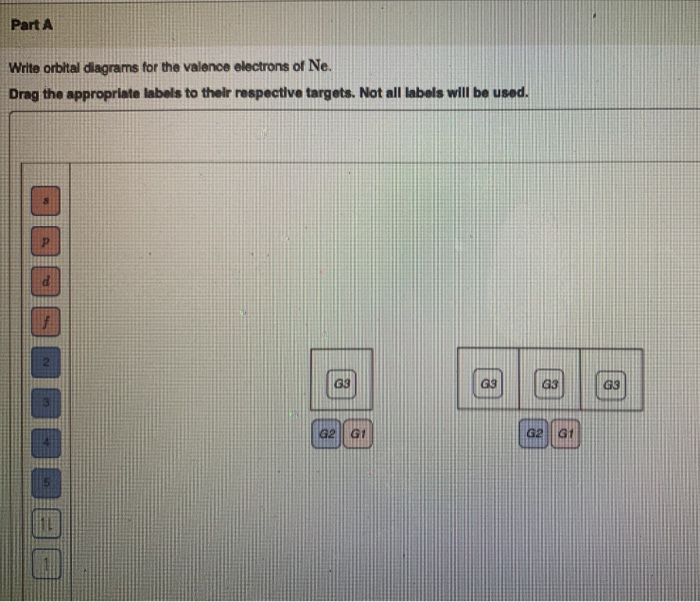
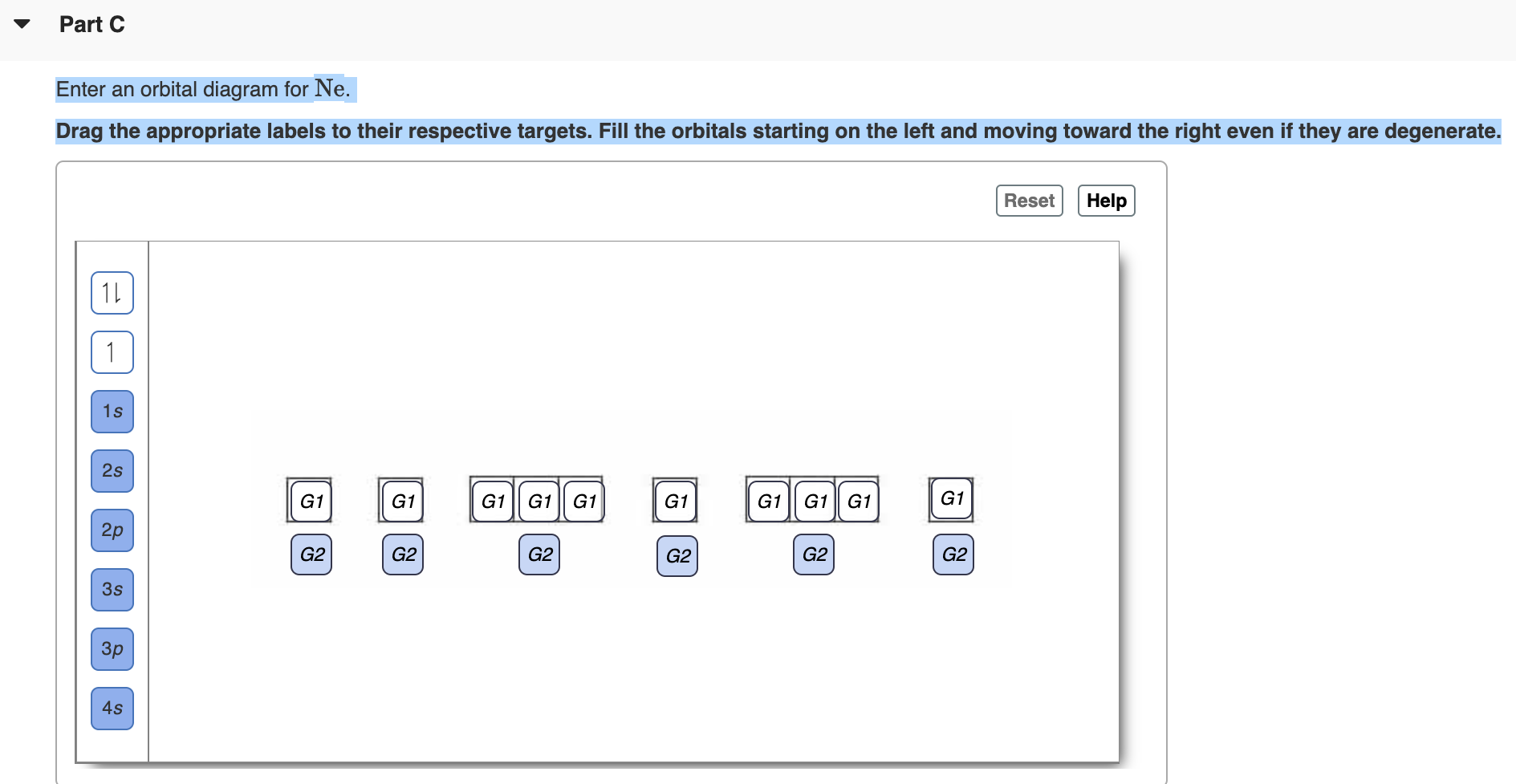
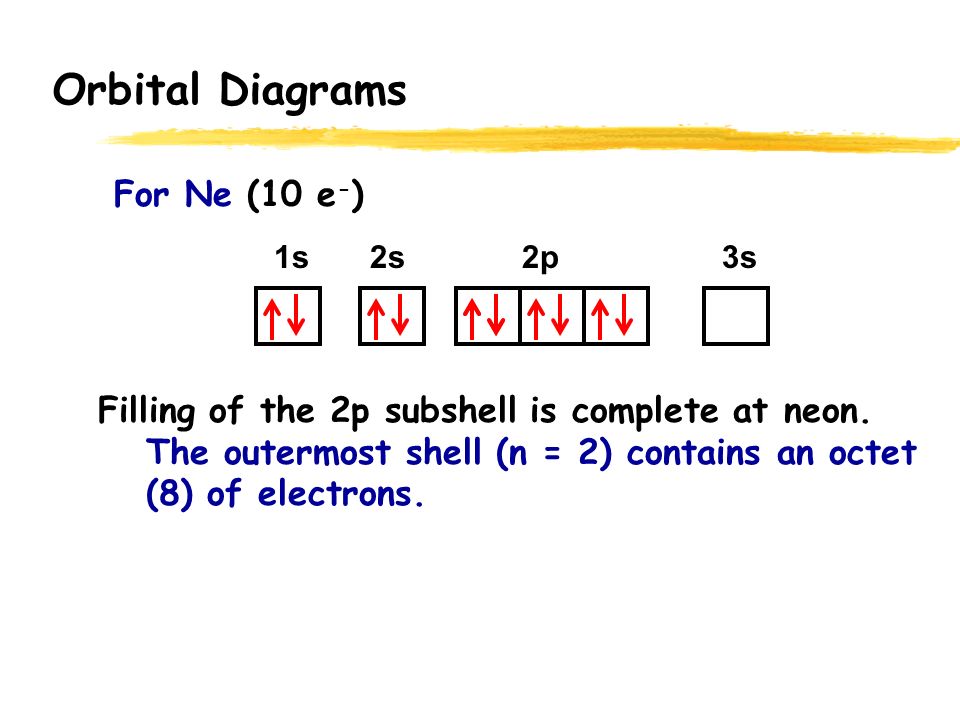
40 full orbital diagram for ne
C has two unpaired electrons in its ground state. The ground-state electron configuration of P is [Ne]3s23p3. The 3s subshell contains one orbital (ml=0), which holds two spin-paired electrons; this subshell is full. The 4p subshell contains three orbitals (ml=-1, 0, 1). Nov 01, 2021 · Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ...
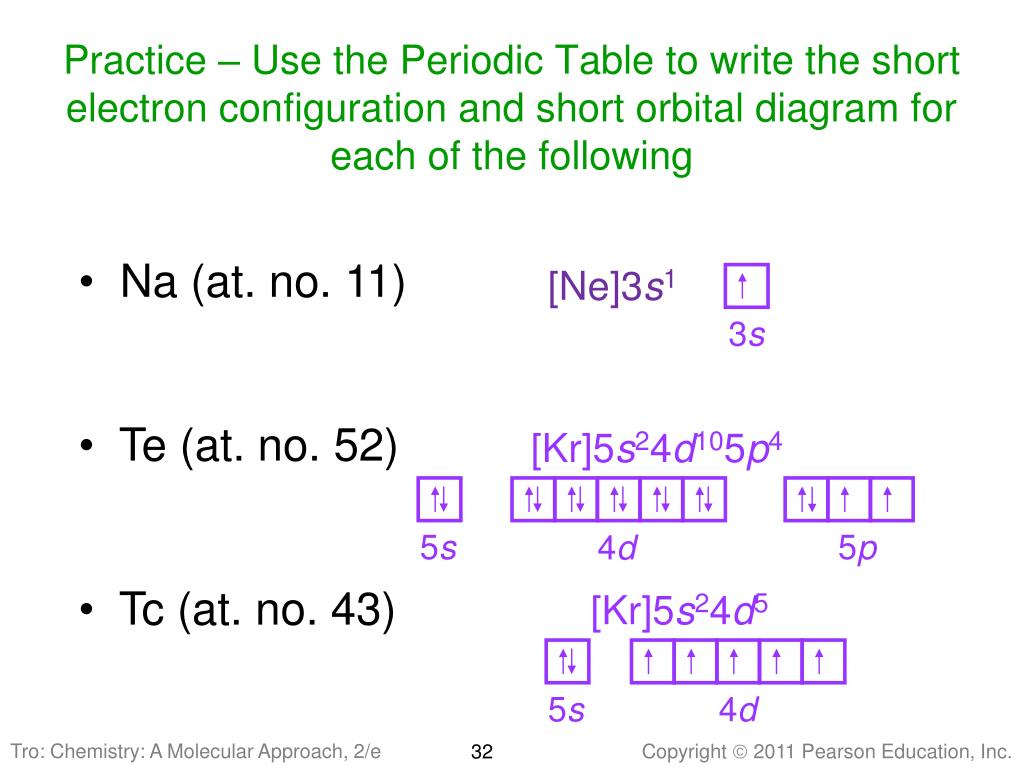
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is ‘Na’. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.
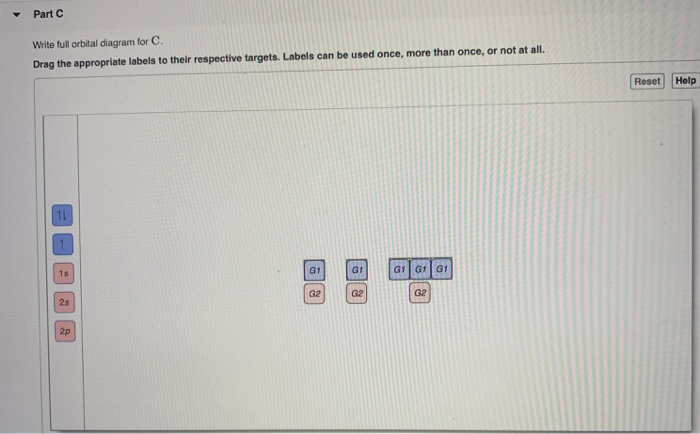
Full orbital diagram for ne
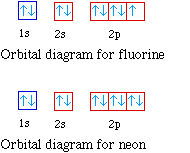
24-09-2019 · Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we’ll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons The simplest kind of orbital is the s orbital, which is spherical in shape, with the nucleus of the atom at its centre. Other orbitals include the p , d and f orbitals. For historical reasons the designated letters stand for sharp , principal , diffuse , and fundamental , based on the spectral line observations associated with them, but the ... Fluorine electron configuration is 1s 2 2s 2 2p 5.The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article.
Full orbital diagram for ne. Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy. For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. 15-08-2020 · Key Terms. Octet rule: A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons. (Hydrogen is excluded because it can hold a maximum of 2 electrons in its valence shell. ) Electron shell: The collective states of all electrons in an atom having the same principal quantum number (visualized as an orbit in which the electrons move). Nitrogen electron configuration is 1s 2 2s 2 2p 3.The period of nitrogen is 2 and nitrogen is a p-block element. The electron configuration of nitrogen(N) and the orbital diagram is the main topic of this article.
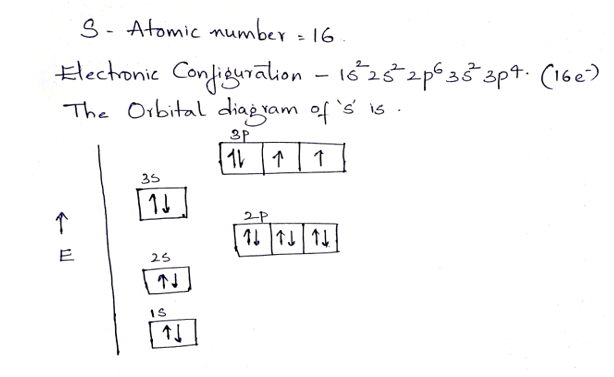
Term symbols with LS coupling. For light atoms, the spin–orbit interaction (or coupling) is small so that the total orbital angular momentum L and total spin S are good quantum numbers.The interaction between L and S is known as LS coupling, Russell–Saunders coupling (named after Henry Norris Russell and Frederick Albert Saunders, who described this in 1925. The molecular orbital energy diagram predicts that He 2 will not be a stable molecule, since it has equal numbers of bonding and antibonding electrons. The Diatomic Molecules of the Second Period. Eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Li 2, Be 2, B 2, C 2, N 2, O 2 ... Oct 01, 2013 · Orbital neoplasms in adults may be categorized on the basis of location and histologic type. Imaging features of these lesions often reflect their tissue composition. Cavernous malformations (also known as cavernous hemangiomas), although not true neoplasms, are the most common benign adult orbital tumor. They typically appear as a well-circumscribed, ovoid intraconal mass on cross-sectional ... 07-11-2021 · Electron Shielding and Effective Nuclear Charge. If an electron is far from the nucleus (i.e., if the distance \(r\) between the nucleus and the electron is large), then at any given moment, many of the other electrons will be between that electron and the nucleus (Figure \(\PageIndex{1}\)). Hence the electrons will cancel a portion of the positive charge of the nucleus and thereby decrease ...
condensed electron configuration: [Ne] 3s 2 3p 6. orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z and 3s boxes, with 6 electrons occupying 3p boxes, that is, each 3p orbital will contain a pair of electrons. Fluorine electron configuration is 1s 2 2s 2 2p 5.The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article. The simplest kind of orbital is the s orbital, which is spherical in shape, with the nucleus of the atom at its centre. Other orbitals include the p , d and f orbitals. For historical reasons the designated letters stand for sharp , principal , diffuse , and fundamental , based on the spectral line observations associated with them, but the ... 24-09-2019 · Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we’ll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons

6 Give The Electron Configuration Use Inert Gas Symbol For Core Electrons And Orbital Diagram For Valence Electrons Homeworklib

Draw The Partial Valence Level Orbital Diagram And Write The Symbol Group Number And Period Number Of The Element Ar 4s 2 3d 10 4p 3 Image Src Orbital9195593143458043682 Jpg Alt Orbital C Study Com

Show The Orbital Diagrams For The Following Chromium Atom And Chromium Iii Ion Include Only Occupied Orbital S A Cr B Cr 3 Study Com


















0 Response to "40 full orbital diagram for ne"
Post a Comment